Figure 1
Age related use of different insulin treatment regimens in children and adolescents.
DOI: https://doi.org/10.4414/smw.2010.13057
Taking care of chronically ill patients, in particular with type 1 diabetes mellitus (T1DM), is a major challenge in paediatrics. This is of great importance as 50% of subjects with T1DM are diagnosed within the first 15 years of life and three quarters of all cases are found in individuals younger than 18 years of age [1]. Furthermore, it has recently been assumed that between 2005 and 2020 the prevalence of T1DM in children younger than 15 years will rise by 70% [2]. In Switzerland, the only and last nationwide survey of the 0–14 year age group revealed an incidence rate of 10.5 per 100’000, with a large annual increase in incidence of 23.8% in children younger than 4 years between 1991 and 1999 [3]. In general, to prevent long-term complications, such as retinopathy, nephropathy as well as neuropathy for example, the best possible metabolic control in both children and adults with T1DM is crucial. Underlining these facts, the Diabetes Control and Complications Trial (DCCT) in 1993 followed by the Epidemiology of Diabetes Interventions and Complications (EDIC) study in 2003 unambiguously showed that improving glycaemic control in a population of 13–39 year old T1DM subjects reduced the risk for microvascular complications [4, 5]. In addition, it has been reported that a memory effect of an improved metabolic control continues over the years [6], and that intensified therapy resulting in better metabolic control also reduces macrovascular complications [7]. This correlates with the recently reported decrease of overall diabetes-related excess mortality in Switzerland over the last two decades [8]. Also in the paediatric age group, it has been shown that good glycaemic control is the most crucial factor for prevention of long-term microvascular complications. [9]. This can be achieved by daily self-monitoring of blood glucose (SMBG) and by regular measurements of glycated haemoglobin (HbA1c), which has a strong predictive value for diabetic complications [10]. According to the guidelines of the American Diabetes Association (ADA), treatment of T1DM should target a HbA1c of below or around 7% in adults, between 7.5% and 8.5% in pre-schoolers, below or around 8% in school aged children and below or around 7.5% in adolescents and young adults [11, 12]. In contrast, the more stringent guidelines of the International Society for Paediatric and Adolescent Diabetes (ISPAD) recommend a target HbA1c level of less than 7.5%, if achieved without severe episodes of hypoglycaemia [13].
Therefore, the aims of this study were a) to assess the quality of care of T1DM subjects followed at the diabetes outpatient clinic at the University Children’s Hospital, Bern, and to compare it with our data obtained ten years ago [14], and b) to compare our data with those of international studies and guidelines of the ADA and ISPAD.
Subjects with T1DM seen during a four month period at the outpatient clinic of the University Children’s Hospital Bern, Switzerland (October 1st, 2007 to January 31st, 2008) were enrolled in an observational, cross-sectional study. Clinical and demographic data, such as insulin regimen, HbA1c, gender, age at diagnosis, diabetes duration, Body Mass Index (BMI; standard deviation score: SDS [15]), pubertal status according to Tanner staging [16] and daily insulin need (expressed as IU/kg body weight/day), were assessed once during the study period. Patients were considered pubertal from Tanner stage 2 onwards [16].
Following a descriptive analysis and after testing the two study populations (1998 vs. 2008) for differences (insulin need, age at diagnosis, diabetes duration, BMI), we compared actual mean HbA1c values obtained with our previously reported data [14] as well as published studies from other centres [17–19], in order to challenge our own treatment strategies.
Concerning diabetes duration, we distinguished between a “honeymoon-group” (diabetes duration of <2 years and/or <0.5 IU insulin / kg) and a group with no residual activity (>2 years duration, C-peptide negative) [20, 21]. The conventional regimen consisted of twice-daily or three-dose insulin injections daily, whereas intensified treatment was defined as either functional insulin treatment (FIT) or continuous subcutaneous insulin injection (CSII). Furthermore, the conventional regimen involves the administration of two or three injections of insulin, mainly a combination of regular short-acting and intermediate-acting insulin (usually before breakfast and dinner, and at bed-time, respectively), coupled with self-monitoring of blood glucose (SMBG) and adjustments of insulin dosage in response to the individual’s glycaemic control. Importantly, hyperglycaemia (>12 mmol/L) was corrected with rapid-acting insulin (for details see: http://www.kinderkliniken.insel.ch/scripte-endo01.html). In FIT treated subjects, however, insulin is applied in a more physiological way partly mimicking the insulin production of the healthy pancreas. This treatment implies taking rapid-acting insulin before each meal and four adjustment injections (bolus) and two long-acting insulin doses to cover the need for insulin between meals and during the night (basis). (http://www.kinderkliniken.insel.ch/scripte-endo01.html)
As there are different types of hypoglycaemia, of which mild and moderate types are often seen particularly in those with a very good metabolic control we asked for and registered the severe hypoglycaemic episodes only. In these episodes, regarded as blood glucose <3.5 mmol/L with loss of consciousness or seizure, severe symptoms of hypoglycaemia disable the child temporarily, requiring the assistance of another person to give something to eat or a glucagon injection.
HbA1c was determined by the Latex-Immunagglutination method (DCA 2000 Analyzer, Bayer Corporation, Elkart, IN 46514 USA). The same Analyzer was used for HbA1c assessment in 1998 and 2007/2008. For this NGSP-certified assay, normative HbA1c values for healthy, non-diabetic individuals range between 4.0% and 5.6%. It allows an accurate measurement for values up to 14%, while higher values are recorded as >14%. Therefore, in order to allow numeric analysis of the data, all values >14% entered the analysis as 14%. This study was approved by the local ethical committee, and oral consent was obtained by either patients and/or parents.
All data are expressed as medians (25th/ 75thcentiles). Due to a lack of normal distribution of our data, statistical analysis was performed applying the Mann-Whitney U-Test using SPSS (Superior Performing Software System; SPSS Inc. Headquarters, 233 S. Wacker Drive, Chicago, Illinois 60606. USA). p <0.05 was considered as statistically significant. Furthermore, despite the small data sets, a multiple linear regression analysis includinginsulin need, BMI (in SDS), age, gender, pubertal stage (Tanner) and duration of diabetes was performed (SPSS).
A total of 152 diabetic patients (88 males, 64 females) entered the study. Their characteristics are described in table 1. To summarise: one third of the patients had diabetes for more than 2 years, and were C-peptide negative; additionally, more than 60% were pubertal, and 66 patients (43.4%) were on a conventional regimen whereas 68 (44.7%) and 18 (11.9%) were treated with FIT and CSII, respectively. Overall median HbA1c was 7.6% (25th/75thcentiles: 7.0/8.3); in more detail, male and female patients presented with a median HbA1c level of 7.6% (6.9/8.3) and 7.7% (7.1/8.5), respectively. It is important to stress that metabolic control in our cohort was not different between the insulin regimen groups. Median HbA1c was 7.5% (6.9/8.3) in patients treated conventionally, whereas in those treated with an intensive regimen median HbA1c was 7.6% (7.1/8.4). Interestingly, while the prevalence of childhood obesity and being overweight in Switzerland is reported to be 15–20%, only one patient (0.65%) presented with a BMI above +2 SDS, and only 5 individuals (3.28%) presented with a BMI > +1.5 SDS highlighting the positive effect of a tight control by a clinical dietician [22].

Figure 1
Age related use of different insulin treatment regimens in children and adolescents.
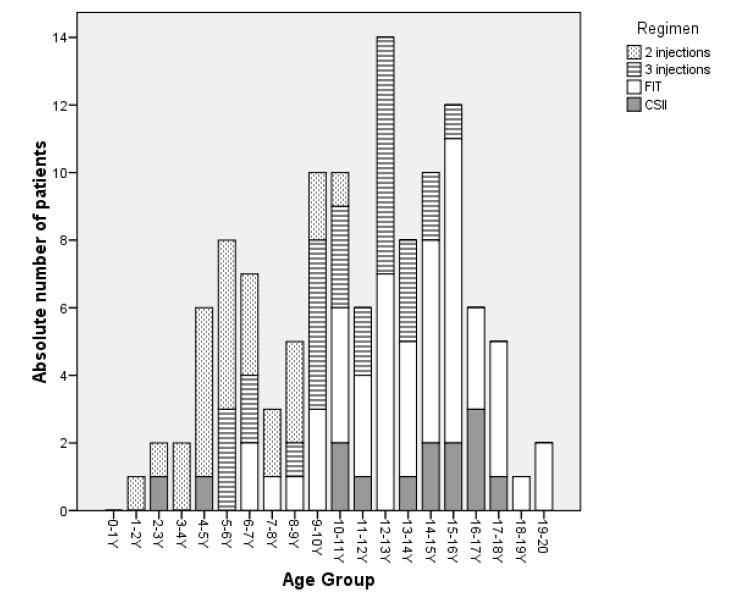
Figure 2
Treatment modalities 1998–2008.
Focusing on the diabetes control, the ADA goals were reached by 56/70 pre-school children (80%), 38/61 of the subjects aged 6 to 12 years (62.3%) and 11/21 of the patients aged 12 to 19 years (52%). However, the more stringent ISPAD goals were only reached by 40/152 subjects (26.3%).
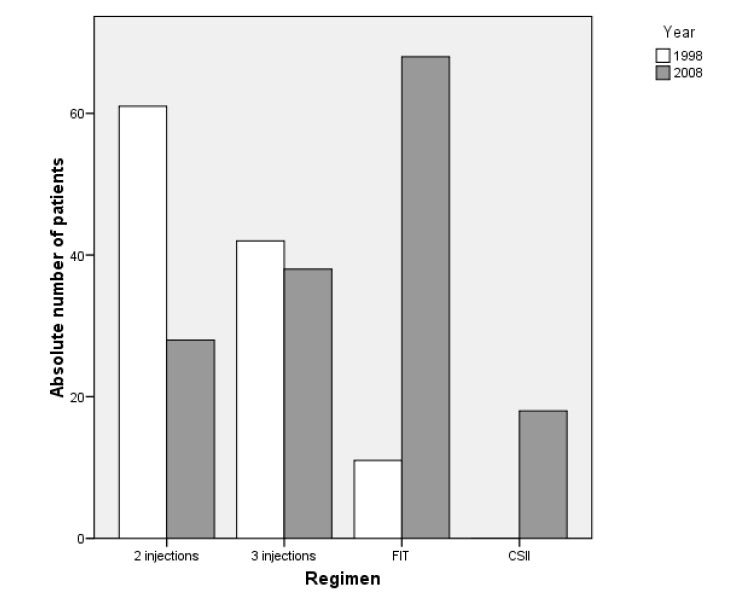
Multiple regression analysis showed that the only variable significantly related to HbA1c was the duration of diabetes (p <0.001), while the other parameters did not show a correlation (data not shown). During the data collection period, only one case of severe hypoglycaemia with unconsciousness and seizures was observed, and this occurred in a patient treated with FIT. In addition, we performed age-related analysis for treatment modalities used in the studied subjects. This analysis revealed a clear prevalence of conventional insulin regimen used during the first 6 years of life (89.5%). This partly explains why the diabetes duration was significantly shorter in conventionally treated subjects compared to the intensive group (1.8 versus 5 years, expressed as median, data not shown). Thereafter, in children older than 6 years, a gradual increase (e.g. 28.5%) in the use of FIT or CSII occurred at the age of 6-7 years, and an 80% increase at the age of 14–15 years was noticed. However, very few toddlers were treated by CSII, while some adolescents did not want to change the treatment and were still managed conventionally (fig. 1).
Our current analysis was compared with the evaluation performed in 1998 [14]. The statistical analysis revealed no significant difference in insulin need, age at enrolment, BMI and diabetes duration between the two study populations (table 2). It is of importance to highlight that the insulin used did not differ at all; the subjects on an intensive insulin regimen in 1998 were already treated with the same insulin analogues. The quality of care evaluation for T1DM at the same outpatient clinic in 1998 revealed a median HbA1c of 7.9% (7.3/8.9). Thus, during the 10 years, metabolic control has significantly (p <0.02) improved (median HbA1c of 7.6%) (table 2). While HbA1c levels were markedly lower in T1DM individuals treated with the 3 injection-regimen in 2008 (p <0.01; HbA1c 1998 vs. 2008), no difference was found for individuals treated with the twice-daily insulin and FIT regimen (p <0.4 and p <0.1, respectively). In addition, over the last decade, treatment of T1DM in children and adolescents revealed a marked shift towards intensified treatment modalities (9.6% vs. 56.5% in 2008, fig. 2).
| Table 1Clinical data, grouped by the pubertal stage, gender, diabetes duration, Body Mass Index and treatment modality, expressed as median (25th/75th). | |||
| Pubertal stage(Tanner) | 1 | 57 (37.5) | 7.4 (6.9/8.1) |
| ≥2 | 95 (62.5) | 7.7 (7.2/8.5) | |
| Gender | male | 88 (57.9) | 7.6 (6.9/8.3) |
| female | 64 (42.1) | 7.7 (7.1/8.5) | |
| Duration | <2 years | 105 (69.0) | 7.6 (6.9/8.2) |
| >2 years | 47 (31.0) | 7.7 (7.0/8.6) | |
| BMI | <1 SDS | 130 (85.5) | 7.6 (7.0/8.3) |
| >1 SDS | 22 (14.5) | 7.8 (7.0/8.4) | |
| Treatment | conventional | 66 (43.4) | 7.5 (6.9/8.3) |
| intensive | 86 (56.6) | 7.6 (7.1/8.4) | |
| Table 2Comparative statistics between the 1998 and 2008 study population. | ||||||
| HbA1c (%, Norm: 4.3–5.6%) | 7.9 (7.3/8.9) | 115 (100) | 7.6 (7.0/8.3) | 152 (100) | < 0.02 | |
| Conventional regimen | 2 injections | 7.80 (7.1/8.5) | 62 (53.9) | 7.7 (7.0/8.3) | 28 (18.4) | <0.4 |
| 3 injections | 8.2 (7.6/8.8) | 42 (36.5) | 7.5 (6.9/8.3) | 38 (25) | <0.01 | |
| Intensive regimen | FIT | 7.9 (7.6/8.6) | 11 (9.6) | 7.6 (7.2/8.5) | 68 (44.7) | <0.1 |
| CSII | 7.5 (6.8/8.2) | 18 (11.9) | ||||
| Age at diagnosis (years) | 6.8 (3.4/11.4) | 6.9 (3.2/10.6) | < 0.6 | |||
| Diabetes duration (years) | 3.2 (1.6/6.0) | 3.4 (1.3/6.5) | < 0.8 | |||
| Insulin dose (IU/kg/day) | 0.9 (0.7/1.0) | 0.9 (0.7/1.1) | < 0.6 | |||
| BMI (SDS) | 0.3 (-0.4/+0.8) | 0.2 (-0.4/+0.7) | < 0.3 | |||
This cross-sectional study shows a significantly improved metabolic control in 152 type 1 diabetics, at the childrens’ and adolescents’ diabetes centre of the University children’s hospital Bern, over the last 10 years. This effect was noted without obvious changes in the characteristics of the population of patients, personnel or methodology of measuring HbA1c. Expressed as mean ± 1SDS, the current HbA1c was 7.8 ± 1.4% compared to 8.0 ± 1.2% in 1998. The most significant difference between 1998 and 2008, as far as the glycaemic control is concerned, was observed in the subgroup treated with 3 insulin injections daily. This might be due to the fact that in the last 10 years, our policy has been towards introducing intensive insulin regimens earlier, partly in order to increase flexibility and reduce diet-related treatment and compliance burdens. The introduction of corrections with insulin analogues, even in conventionally treated subjects, could also partly explain this improvement. Furthermore, the significantly (p <0.001) shorter duration of diabetes in the subgroup on conventional insulin therapy may play an important role (1.8 years vs. 5 years: conventional vs. intensive), as the multiple regression analysis showed that the only factor having a positive impact on a better metabolic control was the duration of diabetes (p <0.001), but not age, insulin regimen or pubertal stage. In our cohort, age-related treatment modalities revealed that the conventional, less invasive treatment was predominantly used in young age, whereas after 6 years of age the number of children using FIT increased constantly.
In terms of weight, underlining the impact of a tight diet control, the occurrence of being overweight or obesity was much lower than in non-diabetic Swiss children.
Furthermore, it is of importance to compare our treatment results not only over the years but also with other international studies and treatment guidelines. For instance, the Hvidøre study group reported, in 1995 and 1998, substantial differences in metabolic control between several paediatric diabetes centres around the world [17, 18]. Additionally, after a decade, no significant improvement in glycaemic control was observed, with mean HbA1c values ranging from 7.4 to 9.2% regardless of the insulin regimen. Overall, in these studies 64% of the female and 56% of the male subjects were on an intensive insulin treatment [19], which was similar to our cohort (2008: 56.3% intensive). Compared to these study centres, we observed a mean HbA1c (±SD) of 7.8 (±1.4), which is significantly (p <0.01) lower than the mean HbA1c of the Hvidøre study (8.2 ± 1.4).
In addition, comparing mean HbA1c levels obtained in the different age groups, pre-school children (7.6 ± 0.7%) and school aged subjects (7.6 ± 1.2%) reached the ADA targets for HbA1c, while the adolescents did not (8.1 ± 1.7%) [11, 12]. In absolute numbers, the subjects reaching the international goals were only between 26.3% (ISPAD) and 52–80% (ADA). However, it is proven that a good metabolic control in patients with T1DM reduces the incidence of complications and delays the progression of existing complications [5]. This, obviously, also holds true for the paediatric population [6]. Therefore, to mimic physiology, intensified insulin replacement modalities (FIT, CSII) may be considered already at an earlier age. On the other hand, the descriptive analysis of our patients treated conventionally or intensively in 2008 showed a similar metabolic control. This may indicate that the insulin regimen may not be the only factor influencing the metabolic control. Adherence to a proposed treatment has been shown to be weaker for intensive regimens, suggesting a mismatch between what clinicians propose and the degree to which patients and their families can manage diabetes [27].
The only severe hypoglycaemic episode was observed in the intensive treatment group. As we did not assess the incidence of mild to moderate hypoglycaemia, commonly occurring in subjects with T1DM on a tight metabolic control, this finding, when it comes to the analysis of hypoglycaemia, should be taken with caution. However it has been shown that an intensive insulin treatment may result in a more stable glycaemic control and cause less mild to moderate and nocturnal hypoglycaemia when compared with conventional regimens [28, 29].
Finally, it is important to individualise the decision of treatment modality for every situation, and a conventional insulin regimen consisting of 2 or 3 insulin injections daily may still be the best and most appropriate therapy for a young child. The choice has to be made together with the patient and his/her parents upon consideration of several factors such as age of the subject, acceptance of restricted versus flexible diet and fixed versus flexible but multiple insulin injections, the cultural/intellectual background of the subject and the family, compliance, and obviously the presence of a partial remission phase.
In conclusion with the current strategy to treat the children and adolescents suffering from T1DM, we almost reached all the guideline-recommended HbA1c targets and are not second to international diabetes centres. Importantly, our patients show a much lower prevalence of being overweight and obesity compared to their non-diabetic peers. Therefore, in our opinion, we have achieved further improvement of the metabolic control in T1DM children and adolescents in the last 10 years through a multidisciplinary approach of care (nurse specialists, psychologists, dieticians, diabetologists) and by the flexible use of all available treatment options on an individual, child friendly base. Nevertheless, there is still a lot to improve to help children to cope with their ever-lasting disease.
1 Vandewalle CL, Coeckelberghs MI, De Leeuw IH, Du Caju MW, Schuit FC, Pipeleers DG et al. Epidemiology, clinical aspects, and biology of IDDM patients under the age of 40 years. Comparison of data from Antwerp with complete ascertainment with data from Belgium with 40% ascertainment. The Belgian Diabetes Registry. Diabetes Care. 1997;20:1556–61.
2 Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltesz G. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet. 2009;373(9680):2027–33.
3 Schoenle EJ, Lang-Muritano M, Gschwend S, Laimbacher J, Mullis PE, Torresani T, et al. Epidemiology of Type I diabetes mellitus in Switzerland: step rise in incidence in under 5 year old children in the past decade. Diabetologia. 2001;44:286–9.
4 DCCT Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86.
5 DCCT Research Group. Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J Pediatr. 1994;125:177–88.
6 DCCT/EDIC Writing Group. Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Development and Complications (EDIC) study. JAMA. 2003;290:2159–67.
7 Nathan DM, Cleary PA, Backlund JY Genuth SM, Lachin JM, Orchard TJ, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–53.
8 Allemann S, Saner C, Zwahlen M, Christ ER, Diem P, Stettler C. Long-term cardiovascular and non-cardiovascular mortality in women and men with type 1 and type 2 diabetes mellitus: a 30-year follow-up in Switzerland. Swiss Med Wkly. 2009;139(39-40):576–83.
9 Nordwall M, Arnqvist HJ, Bojestig M, Ludvigsson J. Good glycemic control remains crucial in prevention of late diabetic complications – the Linköping Diabetes Complications Study. Pediatr Diabetes. 2009;10(3):168–76.
10 Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with microvascular and macrovascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–12.
11 American Diabetes Association. Standards of Medical Care in Diabetes 2009. Diabetes Care. 2009;32:Suppl 1.
12 Silverstein J, Klingensmith G, Copeland K, Plotnick L, Kaufman F, Laffel L, et al. American Diabetes Association. Care of children and adolescents with type 1 diabetes: a statement of the American Diabetes Association. Diabetes Care. 2005;28(1):186–212.
13 Donaghue K, Chiarelli F, Trotta D, Allgrove J, Dahl-Jorgensen K. ISPAD Clinical Practice Consensus Guidelines 2009. Microvascular and macrovascular complications associated with diabetes in children and adolescents. Pediatr Diabetes. 2009;10(Suppl 12):195–203.
14 Flück CE, Kuhlmann BV, Mullis PE. Metabolic control in children and adolescents with diabetes mellitus type I in Berne: a cross-sectional study. Schweiz Med Wochenschr. 1999;129(44):1650–5.
15 Cole TJ, Bellizzi MC, Flegal KM, Dietz W. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320(7244):1240–3.
16 Tanner JM, Whitehouse RH. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch Dis Child. 1976;51(3):170–9.
17 Mortensen HB, Robertson KJ, Aanstoot HJ, Danne T, Holl RW, Hougaard P et al. Insulin management and metabolic control of type 1 diabetes mellitus in childhood and adolescence in 18 countries. Hvidøre Study Group on Childhood Diabetes. Diabet Med. 1998;15(9):752–9.
18 Danne T, Mortensen HB, Hougaard P, Lynggaard H, Aanstoot HJ, Chiarelli F et al. for the Hvidøre Study Group on Childhood Diabetes. Persistent differences among centers over 3 years in glycemic control and hypoglycemia in a study of 3,805 children and adolescents with type 1 diabetes from the Hvidøre Study Group. Diabetes Care. 2001;24(8):1342–7.
19 De Beaufort CE, Swift PG, Skinner CT, Aanstoot HJ, Aman J, Cameron F et al. Continuing Stability of Center Differences in Pediatric Diabetes Care: Do Advances in Diabetes Treatment Improve Outcome? The Hvidøre Study Group on Childhood Diabetes. Diabetes Care. 2007;30(9):2245–50.
20 Lombardo F, Valenzise M, Wasniewska M, Messina MF, Ruggeri C, Arrigo T, et al. Two-year prospective evaluation of the factors affecting honeymoon frequency and duration in children with insulin dependent diabetes mellitus: the key-role of age at diagnosis. Diabetes Nutr Metab. 2002;15(4):246–51.
21 Böber B, Dündar B, Büyükgebiz A. Partial remission phase and metabolic control in type 1 diabetes mellitus in children and adolescents. J Pediatr Endocrinol Metab. 2001;14(4):435–41.
22 Bovet P, Chiolero A, Paccaud F. Epidemiology and prevention of obesity in children and adolescents. Rev Med Suisse. 2008;4(148):650–2, 654–6.
23 Guerci B, Benichou M, Floriot M, Bohme P, Fougnot S, Franck P, et al. Accuracy of an electrochemical sensor for measuring capillary blood ketones by fingerstick samples during metabolic deterioration after continuous subcutaneous insulin infusion interruption in type 1 diabetic patients. Diabetes Care. 2003;26(4):1137–41
24 Acerini CL, Cheetham TD, Edge JA, Dunger DB. Both insulin sensitivity and insulin clearance in children and young adults with type I (insulin-dependent) diabetes vary with growth hormone concentrations and with age. Diabetologia. 2000;43:61–8.
25 Dunger DB, Edge JA. Diabetes and endocrine changes of puberty. Pract Diab Internat. 1995;12:63–6.
26 Du Pasquier-Fediaevsky L, Chwalow AJ, Tubiana-Rufi N. Is the relationship between adherence behaviours and glycaemic control bi-directional at adolescence? A longitudinal cohort study. Diabet Med. 2005;22(4):427–33.
27 Hood KK, Peterson CM, Rohan JM, Drotar D. Association Between Adherence and Glycemic Control in Pediatric Type 1 Diabetes: A Meta-Analysis. Pediatrics. 2009;124;1171–9.
28 Chase HP, Dixon B, Pearson J, Fiallo-Scharer R, Walravens P, Klingensmith G, et al. Reduced hypoglycemic episodes and improved glycemic control in children with type 1 diabetes using insulin glargine and neutral protamine Hagedorn insulin. J Pediatr. 2003;143:737–40.
29 Murphy NP, Keane HN, Ong KK, Ford-Adams M, Edge JA, Acerini CL, et al. Randomized cross-over trial of insulin glargine plus lispro or NPH insulin plus regular human insulin in adolescents with type 1 diabetes on intensive insulin regimens. Diabetes Care. 2003;26:799–804.
No external funding. No competing interests.