DOI: https://doi.org/10.4414/smw.2010.13069
Starting February 2009, an outbreak of respiratory disease took place in Mexico; only weeks afterwards, a novel influenza – the A/H1N1v virus – was detected as its cause. In April 2009, the first cases were detected in the United States. Other regions, including Europe, were soon confirming cases, and on 11 June 2009 the WHO announced a phase 6 pandemic.
In Switzerland the first A/H1N1v patient was diagnosed in late April 2009; but cases reached the national epidemic threshold only in late October (week 43). The culmination point occurred in end-November. End of February 2010, cases dropped below the national epidemic threshold [1].
Worldwide, the overwhelming majority of persons infected with the new H1N1 virus experienced uncomplicated influenza-like illness, with full recovery within a week even without medical treatment. This was stated in a WHO overview [2] and emphasised in various national and international reports [1, 3–4]. Nevertheless, severe complications, including death, have been reported here and elsewhere [2, 5].
The WHO identified the following groups as being at elevated risk of complicated disease (children’s risk groups excluded): pregnant women, persons of any age with chronic pulmonary/ cardiac/ renal/ hepatic disease, with metabolic disorders (e.g. diabetes), certain neurological conditions, haemoglobinopathies, immunosuppression, and persons aged 65 and over. In the course of the pandemic obesity evolved as an additional risk factor [2, 6–14].
The WHO estimated that about half of hospitalised patients (two thirds of patients admitted to ICU) had one or more underlying medical conditions [2].
The most frequent serious complications of influenza are pulmonary [15], with a small subset of patients rapidly developing severe progressive pneumonia [2]. According to WHO reports, primary viral pneumonia is the most common finding in severe cases and the most prominent cause of fatal outcomes. A broad range of secondary bacterial infection rates have also been cited (e.g. 3.1% [11] and 20.3% [12] for ICU patients; 29% [16] and 30% [2] for fatal cases). Respiratory failure and refractory shock are the most common causes of death [2].
During the pandemic various countries reported courses involving severe progressive pneumonia [4–14, 17]. Among these, Australia and New Zealand reported an unusual number of young patients with ARDS requiring extracorporeal membrane oxygenation [18]. Death rates due to A/H1N1v-associated pneumonia based on these reports varied from 14.3% [10, 12] to 39% [17], with the reservation that different groups were described (partly including children, partly with restrictions for intensive care patients).
We investigated the clinical course of patients hospitalised with A/H1N1v-associated pneumonia in a regional hospital in Switzerland.
The study was conducted at the St. Gallen Cantonal Hospital, a tertiary care center serving a population of approximately 500 000, with primary care facilities for 100 000. Starting in May 2009, an influenza subunit was installed within the emergency ward. During the pandemic the centre also served as the referral hospital for A/H1N1v patients with severe complications. In patients with flu-like symptoms who presented at our institution, symptoms were recorded using a structured database system. If the patients qualified according to an algorithm provided by the Swiss Federal Office of Public Health (SFOPH), nose and throat swabs were taken. In the local microbiology laboratory the Roche RealTime ready influenza A/H1N1 detection set was used, with simultaneous PCR detection of influenza A and the specific pandemic H1 gene.

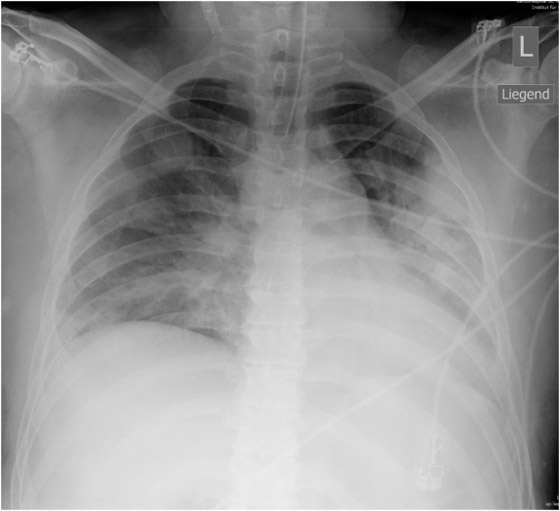
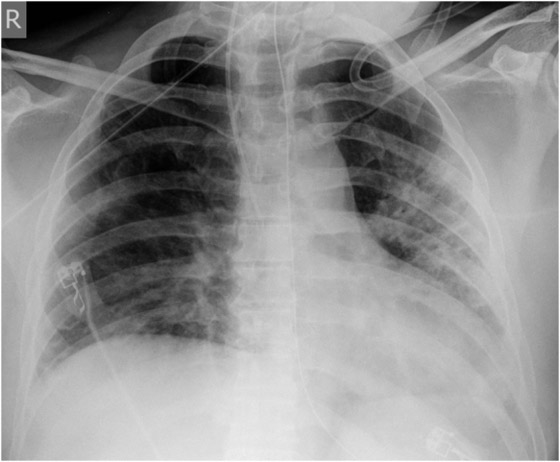
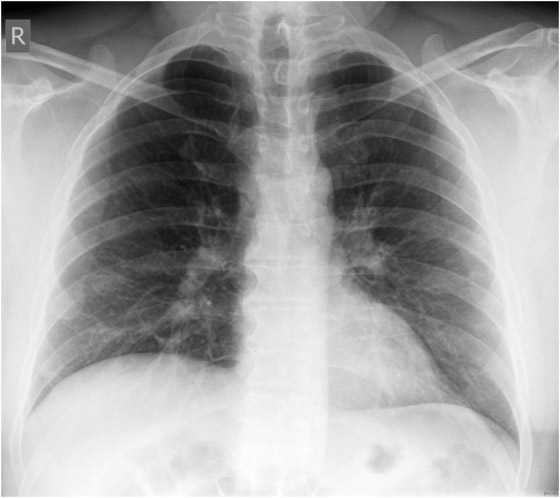
Figure 1
X-ray course of patient A. Left, normal chest x-ray (p.a., erect) at admission. Center, chest x-ray (p.a., supine) on day 4, showing massive infiltration. Right, chest x-ray (p.a., supine) day 14, after 10 days on oseltamivir and 5 days on steroids, showing regression of infiltration.
Essentially, the SFOPH algorithm indicated testing in cases of underlying risk factors, contact with persons with underlying risk factors, or need for hospitalisation. Chest x-rays, laboratory tests and other investigations were performed as mandated by the clinical condition.
Within seven weeks (13 November – 31 December 2009) a total of 15 patients with both A/H1N1v-virus infection and pneumonia were identified from a total of 54 patients hospitalised with confirmed A/H1N1v infections. 11 of these patients had presented themselves in our hospital, 4 patients had been transferred from other hospitals.
This study is a case series including all patients treated at our hospital for A/H1N1v-associated pneumonia, all of whom were treated in November and December 2009. A standardised computer-based form including demographic data, underlying medical conditions, clinical signs, symptoms, treatment and outcomes was completed by physicians in the infectious diseases department, with further data collected via regular follow-up of hospitalised patients by infectious disease specialists and attending physicians. Laboratory and radiological data were also included.
The median patient age was 43 years (30–82), two thirds were male and all Caucasian. The most prevalent preceding symptom (table 1) was cough (100%). Thirteen patients reported fever around or above 39°C lasting a median five days. The two patients without reported fever were over 65 years old; one of these had felt feverish; the other was on low-dose steroid therapy and had experienced chills. Dyspnoea was reported by 80% (n = 12) of the patients, of whom nearly all showed abnormal oxygen saturation (table 1). Nearly 80% (n = 11) of the patients had abnormal pulmonary findings on auscultation. Chest x-rays showed changes in 1-4 (median 2) quadrants (table 1). In 12 patients the positive A/H1N1v PCR test was obtained during hospitalisation; in 3 patients the test had been done 1–4 (median 2) days before.
Of the 15 patients only 8 (53%) presented preexisting risk factors: COPD Gold stage IV (n = 2; one with hypertensive and valvular cardiopathy); bronchial asthma (n = 2; one with additional diabetes mellitus, CVI, and obesity Grade I); myotonic dystrophy (Curschmann-Steinert disease) (with obesity Grade II) and Grade I-II obesity (n = 3). Of the seven patients without WHO-identified risk factors, one had been diagnosed with obstructive sleep apnoea syndrome (OSAS); OSAS was subsequently diagnosed in another obese patient and was suspected in a third. Three patients were smokers and one aged 82 had developed nosocomial A/H1N1v-associated pneumonia after spinal surgery. No patients were pregnant or known to be HIV positive.
Laboratory results at entry are given in table 1. Most patients had lymphopenia and all presented elevated CRP counts (median: 83 mg/L).
Fourteen patients were hospitalised. The only patient who could be treated on an outpatient basis had had influenza symptoms for 10 days and had started oseltamivir treatment two days before presentation. In 79% of the patients studied oseltamivir was initiated at entry (table 2). At this time they had already presented influenza symptoms for a median seven days (table 1). After oseltamivir was started it took a median four days for the patients to become afebrile (table 2).
Only 3 patients, seen during the second half of the pandemic wave, received oseltamivir within 2 days of the appearance of symptoms. One of these had pre-morbid pulmonary disease; one had acquired the infection nosocomially.
Investigating the treatment delays affecting the 12 remaining patients, we found that 4 had deferred presentation, while 1 had initially declined oseltamivir treatment. In the final 7 cases oseltamivir treatment had initially been withheld by the attending physician. Reported reasons for this included: initially mild (2), atypical (no fever, 1) presentation of the disease; clerical error (1); possible deferment by the family doctor (1); medical doubts concerning oseltamivir efficacy after several days of disease (2). In some of these waiting for antibiotic effects caused (overlapping) delay.
During hospitalisation the disease course varied widely (table 2), from relatively mild disease with a hospital stay of three days, to long hospital stays of 35 and 36 days including complications. Six of the patients required intensive care, three with intubation.
No patient died. Three patients left hospital with home oxygen therapy apparatus due to respiratory insufficiency. In one of these, a patient with very advanced COPD, previous home oxygen therapy was continued. In two other patients with very advanced COPD or muscular dystrophy (Curschmann-Steinert disease), BiPAP home oxygen therapy equipment was installed. It is difficult to say to what extent A/H1N1v and the associated pneumonia aggravated these preexisting conditions, and whether the limitation will result in chronic oxygen dependence.
Three patients had additional complications:
Patient A (male, 41 years), with OSAS, was hospitalised for fever and general symptoms with proven A/H1N1v infection. At that time he had leukocytosis (13 g/L), lymphopenia (11%) and a CRP count of 113 g/L, but his lung tests and chest x-ray remained normal (fig. 1a). Four days later the patient rapidly developed respiratory insufficiency requiring intubation. At this time bilateral coarse rales were heard on pulmonary auscultation and the chest x-ray showed diffuse bilateral pulmonary infiltrates (fig. 1b). Oseltamivir was started only then. The disease course was highly complicated with the longest duration of hospitalisation in this series (36 days, including 19 days in intensive care, 13 days intubated). Figure 1c shows slow recovery on day 14 after 10 days of oseltamivir and 5 days of steroids. Recovery was further complicated by generalised weakness requiring admission to a rehabilitation unit.
Patient B (male, 40 years, no pre-existing disease) had received oseltamivir on day 9 of illness, after he had already complained of dyspnoea for 8 days. He had a difficult disease course with hospitalisation for 18 days (11 in intensive care, 3 days intubated). Late in the disease he became symptomatic with pancreatitis (laboratory-proven, but without changes on ultrasound or computed tomography), which was interpreted as either a symptom of A/H1N1v infection or a side-effect of pharmacological treatment (high dose oseltamivir, 150 mg/bid). He also developed clinical signs of pericarditis without echocardiographic changes. Both conditions subsided uneventfully.
Patient C (female, 67 years, advanced COPD and valvular and hypertensive cardiopathy) was hospitalised two weeks before admission for bacterial pneumonia which responded well to antibiotics. When developing A/H1N1v-associated disease with diffuse infiltrates, leukocytosis and CRP of 253 mg/L, but no fever, she developed heart failure and recurrent tachycardia. Due to a clerical error, oseltamivir was started only on day 14 after onset of symptoms; by this time the symptoms had already improved.
Of the nine patients who received steroids (table 2), the indication and timing differed: in four patients (two with bronchial asthma and two with COPD), steroids were initiated at hospital admission to mitigate the underlying condition. In the five remaining patients, steroids were started in two (patients A and B) when ARDS developed, and in the other three when ARDS seemed impending (infiltrates in all lung quadrants and, in two, bloody sputum). In nearly all patients (n = 13), antibiotics were given (table 2).
| Table 1Preceding symptoms, signs, x-ray and laboratory at presentation / hospitalisation in all 15 patients with pneumonia. | |
| Preceding symptoms | Cases (%) |
| Cough | 15 (100%) |
| Fever documented at home | 13 (87%) |
| Fever duration, days * | 5 (2–10) |
| Dyspnoea | 12 (80%) |
| new | 8 |
| preexisting, worsening | 4 |
| Back or limb pain | 7 (47%) |
| Headache | 9 (60%) |
| Gastrointestinal symptoms | 3 (20%) |
| At presentation / hospitalisation | |
| Rales on auscultation | 10 (71%) (one patient: new during hospitalisation) |
| Rales on both sides | 6 (41%) |
| Only wheezing on auscultation | 1 (7%) |
| X-ray, no. of positive quadrants* | 2 (1–4) |
| Laboratory values* | |
| Oxygen saturation** | |
| normal | 2 (13%) |
| abnormal*** | 11 (73%) |
| Haemoglobin, G/L | 14 (10.2–16.2) |
| Leukocytes, G/L | 5.6 (3.2–21.8) |
| Lymphocytes, G/L | 0,9 (0.5–2.5) |
| Neutrophil granulocytes, G/L | 6,6 (1.5–19.3) |
| Platelets, G/L | 213 (114–361) |
| C-reactive protein values, G/L | 83 (26–323) |
| AST (<40), U/l | 42 (17–121) |
| ALT (<55), U/l | 33 (8–57 ) |
| LDH (<265), U/l | 370 (185–1011) |
| CK (<170), U/l | 141 (73–4440) |
| * median (range)** oxygen saturation not measured in two patients who clinically appeared to have normal oxygenation*** oxygen saturation <94%; arterial pO2 <72 mm Hg, including 4 patients experiencing worsening of oxygenation with preexisting deficit | |
| Table 2Treatment and disease course in 14 hospitalised patients with pneumonia. | |
| Hospitalisation | N (%) |
| Directly at our hospital | 9 (64%)* |
| Transfer from other hospital | 4 (29%) |
| Transferred to regional hospital | 1 (7%) |
| Oseltamivir given | all |
| Oseltamivir started at entry* | 11 (79%) |
| Oseltamivir given later during hosp. | 3 (21%) |
| Time from symptom start to oseltamivir, days** | 7 (1.5–14) |
| Duration from oseltamivir start to afebrile, days** | 4 (2–15) |
| Oseltamivir “double dose” (2 x 150 mg) | 12 (80%) |
| Oseltamivir treatment duration | 7 (3–12)** |
| Steroids given | 9 (64%) |
| Antibiotics given | 13 (93%) |
| Duration of hospitalisation, days** | 8–9 (3–36) |
| Death | |
| Complications | |
| Intensive care | 6 (43%) |
| Intubation | 3 (21%) |
| Duration, days | 3/4/13 |
| ARDS criteria fulfilled | 2 |
| Use of vasopressors | 2 |
| * one case developed a nosocomial infection** median (range) | |
This study reports on 15 patients with A/H1N1v-associated pneumonia from one clinical centre. Except for one patient who received outpatient oseltamivir treatment two days before admission, none had received antiviral treatment prior to admission. Eight of the patients had symptoms that increased their risk of developing severe or complicated disease. According to WHO strategy, in these patients oseltamivir treatment should have been “initiated as soon as possible following onset of illness” [19].
Additionally, WHO-defined criteria indicate a complicated course of A/H1N1v disease: “persistent high fever and other symptoms beyond three days” and/or “clinical presentation (e.g., shortness of breath/dyspnoea, tachypnoea, hypoxia)” [19]. In our patients the median duration of fever before hospitalisation was five days. Of the eight patients who gave details on temperature, seven reported measurements of 39°C and above; five cases had been at or above 40.0 °C, equivalent to “high fever”. Most patients had reported dyspnoea before hospitalisation, underlined by changes in oxygen saturation, auscultation findings and abnormal values for blood count and/or CRP. Thus the aforementioned WHO criteria were fulfilled in all cases.
WHO guidelines state that “earlier treatment is associated with better outcomes” [19]. This was suggested by observational data from the United States and Mexico [9, 20, 21], where oseltamivir treatment was started within two days of symptom onset. If treated as early as possible according to these WHO guidelines, those of our patients with risk factors qualifying for oseltamivir treatment at the start of influenza symptoms might not have developed pneumonia at all. Even if pneumonia was not, in fact, avoidable in every case, its clinical course, with a median hospital stay of 8–9 days and 6 patients transferred to intensive care, including intubation in 3 (one for 13 days), might have been milder.
In contrast to early therapy, the effect of treatment with oseltamivir at late stages still remains speculative. In seasonal influenza, parallel to the above findings, an early start to oseltamivir treatment reportedly prevents complications [15, 22], although the value of these data has recently been disputed [23, 24]. However, even >48 hours into the illness, use of oseltamivir still showed a survival benefit in a Canadian study [25]. For H5N1 influenza a survival benefit could be shown even with an oseltamivir start 6–8 days after the onset of symptoms [26]. No clinical trials yet exist for anti-influenza medications against A/H1N1v; however, in one of the first studies from Mexico receipt of a neuraminidase inhibitor was associated with survival independent of the time of treatment start [21]. Based on this and similar experience, in November 2009 the WHO recommended that antiviral treatment for influenza A/H1N1v “may also be used at any stage of active disease when ongoing viral replication is anticipated or documented” [19].
However, in our patients oseltamivir treatment was started very late – median seven days after symptom onset. It is difficult to judge whether, initiated so late, oseltamivir still had any effect, i.e. whether viral replication had already ended. Although no patients died, it took a median four days for the patients to become afebrile, which might have been the disease’s natural course.
Above, we summarised the reported reasons for the observed delays in oseltamivir treatment. However, we believe that this delay was also supported by the initial widespread assumption (also reported in various media) that the clinical presentation of pandemic influenza was mild – even milder than seasonal influenza. Interestingly, all 3 patients whose oseltamivir treatment started within 2 days of A/H1N1v symptom onset were seen during the second half of the study period. We regard this as an indication of a learning curve, especially among medical personnel. Knowledge concerning the high worldwide morbidity and mortality rates of the first wave of pandemic influenza 2009, particularly in young individuals, had probably increased by then.
The WHO recommended higher doses of oseltamivir and a longer duration of treatment “in patients with severe or progressive illness not responding to standard treatment” [19]. In most instances we used oseltamivir double dose and adapted the duration of treatment to the clinical course. Except for patient B (who showed clinical signs of pancreatitis, possibly due to oseltamivir), we observed no adverse effects.
The rationale for steroid use to treat or prevent ARDS is underlined by a report from Argentina. The authors concluded that “in ARDS patients with and without confirmed H1N1 influenza, prolonged low- to moderate-dose corticosteroid treatment was well tolerated and associated with significant improvement in lung injury” [27].
OSAS is not mentioned as an individual risk factor in international (WHO) or Swiss (SFOPH) recommendations, but has been associated with poor outcomes [7, 17]. The prevalence of OSAS is increased in obese people [28]. OSAS was confirmed in two of our patients (including patient A [who was not obese]) and suspected in a third. However, it is conceivable that underreported OSAS may contribute to A/H1N1v morbidity and mortality in obese people.
Except for a very small number of cases where data were insufficient to rule out secondary bacterial pneumonia, primary viral pneumonia was suspected in all cases. Our diagnosis was based on chest x-rays (corresponding to the literature [17, 29]: patchy infiltrates or ground-glass opacities, often bilateral); negative blood cultures and/or sputum or bronchial lavage; lack of response to antibiotic therapy [4 patients had started antibiotics 3–10 days prior to presentation]; and negative procalcitonin in 5 patients. Therefore, inadequate antibiotic treatment was frequent due to the severe or unforeseeable clinical course in most cases.
In this report the proportion of patients with pneumonia among all hospitalised A/H1N1v patients (14/54, 26%) was significantly lower than the corresponding rate presented by the SFOPH (205/571, 36%) [30]. The most likely explanation for this difference is underreporting of hospitalised patients with A/H1N1v without pneumonia to the SFOPH. Nonetheless, pneumonia is certainly the most important complication. This is also reflected by the 20reports to the SFOPH of A/H1N1v-related deaths, of which 13 listed pneumonia as the cause of death [30, 31].
In our series of 54 hospitalised patients with confirmed A/H1N1v infection, other complications included dyspnoea (without pneumonia) and asthma exacerbations. These and other more rarely observed problems, including sepsis, dehydration and fever convulsions, were also reported to the SFOPH by other centres [30]. At our hospital, no patient with A/H1N1 influenza died.
This study reports on 15 patients with A/H1N1v-associated pneumonia. In spite of risk factors for severe A/H1N1 disease (eight patients) and signs and symptoms of progressive disease (all patients), oseltamivir treatment was started only a median six days (i.e. on day 7) after symptom onset. Of the 15 patients studied, 14 had to be hospitalised, with a median stay of 8–9 days; 6 needed intensive care, 3 with intubation. Early oseltamivir treatment according to WHO recommendations might have prevented the development of pneumonia, or, in the case of patients without preexisting risk factors, might have mitigated the disease course.
The authors wish to express their gratitude to Dr. Patrick Mathys, Swiss Federal Office of Public Health, for his valued contributions regarding the epidemiology of influenza A/H1N1v in Switzerland.
1 Swiss Federal Office of Public Health. http://www.bag.admin.ch/influenza/06411/index.html?lang=de
2 WHO. http://www.who.int/csr/disease/swineflu/notes/h1n1_clinical_features_20091016/en/index.html
3 Gilsdorf A, Poggensee G, on behalf of the working group pandemic influenza A(H1N1)v. Influenza A(H1N1)v in Germany: the first 10 000 cases. Euro Surveill. 2009;14(34):pii=19318.
4 Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–15.
5 ECDC. http://ecdc.europa.eu/en/activities/surveillance/EISN/Newsletter/100129_EISN_Weekly_Influenza_Surveillance_Overview.pdf
6 Gomez-Gomez A, Magana-Aquino M, Garcia-Sepulveda CA, Ochoa-Perez AR, Falcon-Escobedo R, Comas-Garcia A, et al. Severe Pneumonia Associated with Pandemic (H1N1) 2009 Outbreak, San Luis Potosi, Mexico. Emerg Infect Dis. 2010;16(1):27–32.
7 Louie J, Winter K, Harriman K, Vugia D, Glaser C, Matyas B et al. Hospitalized patients with novel influenza a (H1N1) infection-California, April-May,2009. MMWR. 2009;58:536–41.
8 Napolitano LM, Park PK, Sihler KC, Papadimos MD, Chenoweth C, Cinti S. Intensive-care patients with severe novel influenza A(H1N1) virus infection – Michigan, June 2009. MMWR. 2009;58(27):749–52.
9 Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit ST, Louie J, et al. Hospitalized Patients with 2009 H1N1 Influenza in the United States, April-June 2009. N Engl J Med. 2009;361:1935–44.
10 Kumar A, Zarychanski R, Pinto R, Cook DJ, Marshall J, Lacroix J, et al (for the Canadian Critical Care Trials Group H1N1 Collaborative). Critically Ill Patients with 2009 Influenza A(H1N1) Infection in Canada. JAMA. 2009;302(17):1872–9.
11 Rello J, Rodriguez A, Ibanez P, Socias L, Cebrian J, Marques A. Intensive care adult patients with severe respiratory failure caused by influenza A /H1N1)v in Spain. Crit Care. 2009;13(5):R148.
12 Webb SAR, Pettilä V, Seppelt I, Bellomo R, Bailey M. Cooper DJ. Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N Engl J Med. 2009;361:1925–34.
13 Cullen G, Martin J, O’Donnell J, Boland M, Canny M, Keane E, et al. Surveillance of the first 205 confirmed hospitalised cases of pandemic H1N1 influenza in Ireland, 28 April – 3 October 2009. Euro Surveill. 2009;14(44):ppi=19389.
14 Yeung JHY, Bailey M, Perkins GD, Gao Smith F. Presentation and management of critically ill patients with influenza A(H1N1): a UK perspective. Critical Care 2009;13:426.
15 Rothberg MB, Haessler SD. Complications of seasonal and pandemic influenza. Crit Care Med 2010;38(3):(Suppl.)
16 Louie J, Jean C, Chen TH, Park S, Ueki R, Harper T, et al. Bacterial coinfections in lung tissue specimens from fatal cases of 2009 pandemic influenza A (H1N1) – United States, May-August 2009. MMWR. 2009/58(38):1071–4.
17 Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, Hernandez M, Quinones-Falconi F, Bautista E, et al. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med. 2009;361:680–9.
18 Davies A, Jones D, Bailey M., Beca J, Bellomo R, Blackwell N, et al. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009;302(17):1888–95.
19 WHO: Clinical management of human infection with pandemic (H1N1) 2009: revised guidance; November 2009. http://www.who.int/csr/resources/publications/swineflu/clinical_management_h1n1.pdf
20 Uyeki T. Antiviral treatment for patients hospitalized with 2009 pandemic influenza A (H1N1). N Engl J Med. 2009;361:e110.
21 Dominguez-Cherit G, Lapinsky SD, Macias AE, Pinto R, Espinosa-Perez, L. Critically ill patients with 2009 influenza A/H1N1) in Mexico. JAMA. 1009;302(17):1880–7.
22 Kaiser L, Wat C, Mills T, Mahoney P, Ward P, Hayden F. Impact of oseltamivir treatment on influenza-related lower respiratory tract complications and hospitalizations. Arch Intern Med. 2003;163:1667–72.
23 Freemantle N, Calvert M. What can we learn from observational studies of oseltamivir to treat influenza in healthy adults? BMJ. 2009;339:b5248.
24 Doshi P. Neuraminidase inhibitors: the story behind the Cochrane review. BMJ. 2009;339:b5164.
25 McGeer A, Green KA, Plevneshi A, Shigayeva A, Siddiqi N, Raboud J, Low DE. Antiviral therapy and outcomes of influenza requiring hospitalisation in Ontario, Canada. CID 2007;45:1568–75.
26 Toovey S. Avex Avian Influenza Expert Group. First results from an avian influenza case registry. Oral presentation at Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) 2009. Abstract V-533, 9/13/2009.
27 Quispe-Laime AM, Bracco JD, Barberio PA, Campagne CG, Rolfo VE, Umberger R, Meduri GU. H1N1 influenza A virus-associated acute lung injury: response to combination oseltamivir and prolonged corticosteroid treatment. Intensive Care Med. 2010;36:33–41.
28 Kono M, Tatsumi K, Saibara T, Nakamura A, Tanabe N, Takiguchi Y, Kuriyama T. Obstructive sleep apnea syndrome is associated with some components of metabolic syndrome. Chest. 2007;131:1387–92.
29 Ajlan AM, Quiney B, Nicolaou S, Müller NL. Swine-origin influenza A (H1N1) viral findings: radiographic and CT findings. AJR. 2009;193:1–6.
30 Swiss Federal Office of Public Health. Hospitalisation reasons and death of confirmed influenza A/H1N1v cases in Switzerland from week 17, 2009, to week 22, 2010. Personal communication (March 18th and June 7th, 2010).
The authors declare no financial or commercial conflict of interest.