Why are we still using counterfoil prescription pads?
Valérie Junod, Carole-Anne Baud, Caroline Schmitt-Koopmann, Jean-Christophe Devaud, Margaux Bonnard, Sophie Pautex, Barbara Broers, Olivier Simon
This article summarises the legal framework of narcotics prescriptions when the use of a counterfoil prescription is mandatory. It outlines supposed benefits and shortcomings of counterfoil prescriptions and recommends replacing them with digital prescriptions.
Introduction
Under Swiss law, counterfoil prescriptions are required whenever a physician prescribes1 a controlled medicine (i.e. narcotic or psychotropic substance)2 belonging to the official schedules a or d3. Schedule a and d substances notably include methadone, morphine, cannabis-based medications and methylphenidate. In contrast, benzodiazepines, z-drugs and codeine, for example, belong to schedules b and c; therefore, an ordinary prescription is sufficient4.
The counterfoil requirement dates back several decades5. It has never been adapted since then. Counterfoil prescriptions also exist, in different forms, in certain foreign countries (e.g. Germany)6. In others, they have been replaced by digital prescriptions7. In this article, we question the relevance of the current Swiss system.
Our article is divided into four chapters. We begin with a description of counterfoil prescriptions and the current legal landscape8. We follow with a critical analysis of the system. This leads us to articulate recommendations and conclude that counterfoil prescriptions should be abandoned.
Counterfoil prescriptions today
The Federal Act on Narcotics and Psychotropic Substances (NarcA9) itself does not mention counterfoil prescriptions (in French, carnets à souches; in German, amtliches Rezeptformular; in Italian, moduli di ricette). Counterfoil prescription rules are based on the Ordinance on Narcotics Control (OCStup10). The OCStup does not use the lay term counterfoil prescription but “ordonnance de stupéfiants prévue à cet effet”11.
When is a counterfoil prescription needed?
As already mentioned, counterfoil prescriptions are only required when prescribing a controlled medicine belonging to schedules a or d. According to art. 46 al. 3 OCStup, controlled medicines belonging to schedules b or c can be prescribed on ordinary prescriptions12. Somewhat surprisingly, veterinary drugs are subject to the same principles13 (art. 50 OCStup). The canton of Jura mentions the possibility of extending the counterfoil prescription requirement to other medicines14, but it has not done so15. The provisions of the OCStup apply regardless of the therapeutic indication. Thus, opioid agonist treatments (OAT) to treat opioid use disorders are encompassed, as is palliative care.
Exceptionally, in cases of medical emergency and if obtaining the physician’s prescription is impossible, the pharmacy can issue the smallest marketed package of the controlled medicine without a counterfoil prescription (art. 52 al. 1 OCStup). However, the pharmacy must document the case and send a report to its cantonal authority. In practice, according to Cantonal Physicians and Cantonal Pharmacists, this is uncommon.
Counterfoil prescription prescribing is accessible to physicians (or veterinary physicians) with a cantonal practice license16, with no further medical requirements (e.g. no need to consult a specialist, no need to administer a special questionnaire, no need to have attended a specific training program). The ordinance does not distinguish between inpatient and outpatient care17, except in the case of animals, whereby counterfoil prescriptions are not required for “inpatient” treatment (art. 50 al. 2 OCStup). However, at least at the Geneva University Hospitals (HUG), the Vaud University Hospitals (CHUV) and the Hôpital du Valais, in practice, counterfoil prescriptions are not used for inpatient care, and in some cases not even for outpatient care when prescribed by hospital doctors. These hospitals use a digital system of secured ordinances, which ensures internal traceability to order controlled medicines from their hospital pharmacy. At the Inselspital in Bern, the counterfoil system coexists with the digital prescription system.
In most of the Swiss cantons, especially in the German-speaking part, propharmacy (physicians dispensing medicines themselves)18 is allowed. Without a specific provision in the OCStup, the dispensing physicians would still be expected to prescribe on counterfoil prescriptions. In practice, propharmacy physicians often do not use a counterfoil prescription, or even write a prescription at all, since they deliver the medicine directly. However, they must be able to account for the dispensed controlled medicines (art. 64 al. 3; 63 OCStup).
What does a counterfoil prescription look like?
Swissmedic, the federal authority for therapeutic products, established the common standard for counterfoil prescriptions. Since 2017, they have used the same trilingual format throughout Switzerland19.
A counterfoil prescription is an A5 paper prescription form, comprising three identical foils (one original and two copies) of three different colours (white, red and blue). Counterfoil prescriptions are combined in pads, which have no expiration date. Each pad has 25 different counterfoil prescriptions. Each counterfoil prescription (i.e. set of three foils) bears a unique number (e.g. 9211351). The numbers within the pad are consecutive. This number is also available through a barcode. The “new” 2017 counterfoil prescription only has space to prescribe two medicines, given the two lines available per counterfoil prescription. Thus, if more controlled medicines belonging to schedules a or d are prescribed, another counterfoil prescription must be used. The paper used for counterfoil prescriptions does not bear transparent marks, but has an anti-copy system in the form of a small, embossed rhombus on the right edge. Since the prescription is hand-written, it is still possible to falsify its content.
The red and blue foils are in carbon paper, allowing two copies of the original (white) prescription. The original white version and the red copy go to the pharmacy, either by post or via the patient20. The white foil is then kept by the pharmacy (art. 63 al. 3 OCStup). The pharmacy sends the red foil to the health insurance company (‘sickness fund’). The blue foil is kept by the physician in their pad or placed in the patient’s medical file (art. 47 al. 4 OCStup). Physicians and pharmacists are to keep their (past) foils for at least 10 years (art. 62 al. 3 OCStup). In cantons with no propharmacy, but where physicians still sometimes deliver substances (e.g. methadone) at their office21, the process is the same: the pharmacy also receives the white foil and the red copy.
The content of the counterfoil prescription is described in art. 47 OCStup. The counterfoil prescription must include “the name, address, signature and stamp of the prescribing physician; the first name, last name, birthday and address of the patient; the date of the prescription; the name of the controlled medicine, galenic form and dosage; the quantity prescribed and the posology”. It must be signed by the physician (art. 47 al. 4 OCStup). This mostly matches the content of ordinary prescriptions (listed in art. 51 al. 1 OMéd22). The differences are minimal, e.g. counterfoil prescriptions must show the patient's address, while this is not true of ordinary prescriptions.
What is the validity of counterfoil prescriptions?
Counterfoil prescriptions have three different limitations of validity.
First, differing from ordinary prescriptions, counterfoil prescriptions are valid only for one month following their issuance (art. 47 al. 2 OCStup). In other words, one month after the prescription has been signed by the physician, the pharmacist will no longer honour it. For ordinary prescriptions, the validity is usually up to one year, but this varies depending on the canton23.
Second, unless otherwise specified by the prescribing physician or veterinarian, the prescription is not renewable (art. 51 al. 3 OCStup). In other words, the pharmacist cannot issue the same medicine twice using the same prescription. In any case, controlled medicines from schedules a and d are classified in catégorie de remise A (ordinance not renewable without the express permission of the doctor – 41 OMéd). In that sense, the OCStup duplicates a rule already valid for ordinary prescriptions.
Third, the amount of controlled medicine prescribed must be limited to the therapeutic need of a one-month period for the given patient (art. 47 al. 3 OCStup). In exceptional cases, counterfoil prescriptions can cover a three-month treatment, provided that this is duly mentioned on the counterfoil prescription (art. 47 al. 3 in fine OCStup). Ordinary prescriptions do not bear such quantitative limitations aside from the aforementioned one-year limit.
Who provides counterfoil prescriptions and for what price?
Federal law states that the cantons are responsible for providing counterfoil prescriptions to physicians and veterinarians (art. 47 al. 5 OCStup). The cantons delegated this task to the Cantonal Physician, the Cantonal Pharmacist or an administrative unit within the cantonal health department. Swissmedic buys the counterfoil prescription pads from the producer at a price between CHF 1.51 and CHF 1.63 (depending on the quantity ordered) per pad and resells them to the cantons for CHF 5 per pad (art. 47 al. 5 OCStup)24. The cantons then either sell or give them free to the physicians (figure 1).
These costs of the counterfoil prescription cannot be charged to the patient, and they are not reimbursed by the sickness fund. Thus, the physician bears the cost.
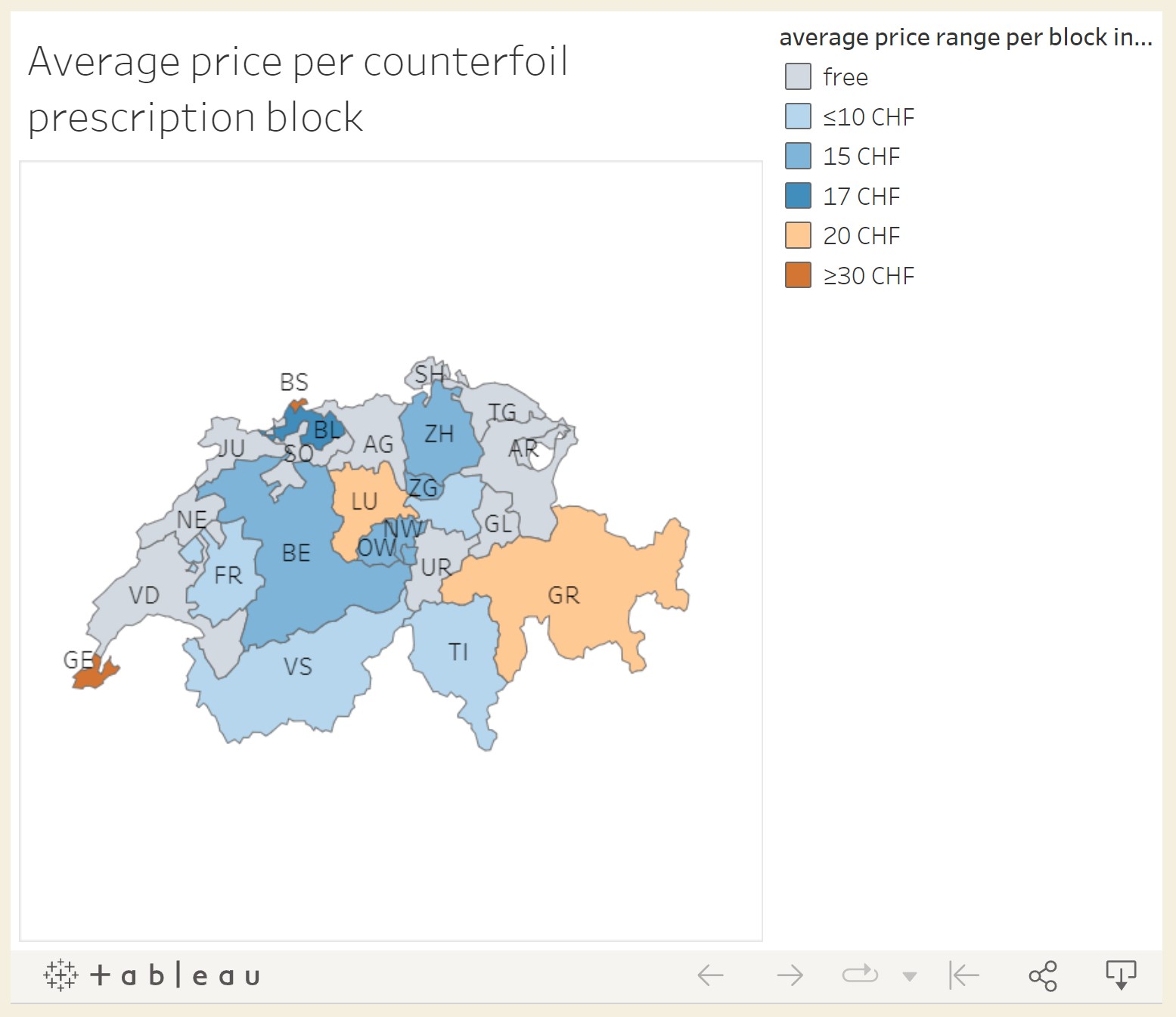
Figure 1: Average price per counterfoil prescription pad.
Interactive map: https://wp.unil.ch/medicaments-sous-controle/projet/cantonal-data/
Table 1. Average price per counterfoil prescription – Data
Canton
Price per PAD [CHF]
Shipping [CHF]
AG
free
free
AR
free
free
BE
5
20
BL
free
50
BS
30
included
FR
7.8
included
GE
30.3
included
GL
free
free
GR
15
10
JU
free
free
LU
20
included
NE
free
free
NW
15
included
OW
15
included
SG
free
free
SH
free
free
SO
free
free
SZ
2.5
30
TG
free
free
TI
7.5 (mind. 10 CHF)
included
UR
free
free
VD
free
free
VS
10
included
ZG
15
included
ZH
15
included
What are the possible objectives and benefits of the counterfoil prescription system?
The legislation does not state the anticipated benefits of the counterfoil prescription. However, one can infer that the system presents the following four advantages:
Reducing falsification
A patient will find it (more) difficult to falsify a counterfoil prescription, as opposed to an ordinary prescription. Ordinary prescriptions are easy to falsify and often are. With counterfoil prescriptions, the patient first needs to obtain an authentic pad, which should be kept securely by the physician. Then, they must falsify (at least) the two copies going to the pharmacy and the sickness fund. However, (re)writing on these carbon foils (e.g. to increase the dosage) is likely to look abnormal to the pharmacist.
Managing stolen, lost or falsified counterfoil prescriptions
A federal mechanism has been introduced to report stolen, lost or falsified counterfoil prescriptions, but without legal basis. First, the physician is supposed to announce such occurrences to the cantonal authority. The latter then forwards them to Swissmedic. The federal authority draws a list of all such counterfoil prescriptions on its website25. In parallel, every week, the Federal Office of Public Health publishes in its bulletin the new blocked counterfoil prescriptions. Finally, before honouring a prescription, pharmacies are supposed to manually check whether the foils that they receive are reported in this list. According to this federal list, from January 2022 to June 2022, at least 620 such occurrences were reported (counting each three-set foil). We do not know how many attempts to obtain a prescription based on a lost, stolen or falsified counterfoil prescription were stopped.
Reducing over-prescription of controlled medicines
By introducing a more burdensome process, the OCStup likely aims to discourage the prescription of controlled medicines, at least when not medically needed. Physicians must go through the hassle of using their special pad. The duration of the prescription is shorter (one month as opposed to one year), meaning that the physician is supposed to meet the patient more often. Physicians, if not acting as propharmacist, can expect more control at the pharmacy level. In some cantons, the authorities track the numbers of counterfoil prescriptions ordered by physicians and ask for an explanation if the orders go above a certain number. Hence, the implicit message is that these medicines should be used with greater care.
Promoting unity
The OCStup creates a certain unity in practice. All counterfoil prescriptions are the same and should be filled in the same manner. Ordinary prescriptions are subject to more discretion, at least regarding their form (e.g. paper, fax, email, digital exchange). Whether, from a legal standpoint, cantons still have leeway in implementing the counterfoil prescription provisions is uncertain, but some have done so. For example, our analysis of cantonal legislation uncovered that Neuchâtel requires physicians to keep counterfoil prescriptions under lock and notify loss or theft without delay and pharmacists to send suspected falsified prescriptions to the Cantonal Pharmacist26. In Jura, counterfoil prescriptions must be registered on a secured registry27.
What are the shortcomings of the counterfoil prescription?
We identify four main shortcomings.
Counterfoil prescriptions are exploited neither to control nor to understand the market
An implicit objective of the counterfoil prescription was to facilitate the control of the market for controlled medicines belonging to schedules a and d (e.g. opioids). In theory, cantonal and federal authorities could thus keep a closer tab on who is prescribing what to whom. For example, the authorities could identify abusive practices (e.g. a sudden spike in the prescription of a certain substance; a physician who prescribes too much of a given medicine) or prevent patients from obtaining multiple prescriptions from different doctors or in different cantons.
However, this has not been the case28. Some cantonal authorities used to request that pharmacies send all their white foils so that the canton could follow what was happening within its territory, but this is obviously difficult with a paper system. Most cantonal authorities have abandoned this practice, which is not required under federal law and rarely formalised under cantonal law. Cantons of German-speaking Switzerland, as well as the cantons of Vaud, Fribourg and Neuchâtel, do not ask to receive counterfoil prescriptions, except possibly during targeted inspections. Jura requests them at the end of the year when it checks the pharmacies’ inventories. Finally, according to their regulations, Vaud and Geneva ask to receive, every month, the counterfoil prescriptions of patients on opioid agonist treatments and (only in Geneva) the counterfoil prescriptions for controlled medicines of the d schedule (e.g. diacetylmorphine)29. Even so, the cantons make no general use of them.
Hence, counterfoil prescriptions are not used to better assess the market, whether in its quantitative or qualitative aspects. We found only two studies performed based on their data30. Counterfoil prescriptions are simply stored and then destroyed.
Only in specific instances of an enquiry against a given physician or pharmacist do the cantonal authorities revert to the counterfoil prescription and ask for justifications. However, they have limited power because they do not have access to the entire patient file; if the physician believes that their prescription is legitimate in the given instance, the authorities find it hard to prove otherwise.
That counterfoil prescriptions do not exist online complicates their use for research or control purposes
Given the sheer number of counterfoil prescriptions in use, entering all the data manually is tiresome, whether for research or control purposes. No one has the time or resources to do so. To control pharmacies’ bookkeeping, the cantonal authorities find it easier to use Swissmedic’s MESA (Meldesammlung für kontrollierte Substanzen) software31. MESA contains all controlled medicine orders by pharmacies and propharmacy physicians, although it says nothing about what medicine was prescribed to whom.
Even physicians and pharmacies would find it hard to use their counterfoil prescriptions to better understand their practice. For example, if a physician would like to know how the dosage of a given patient has evolved, they would have to find all blue foils in the archives and analyse them one by one.
Counterfoil prescription efficacy against falsification or theft is questionable
Counterfoil prescriptions are still often stolen, lost and probably falsified.
That counterfoil prescriptions cannot be transmitted online (even using secured communication tools) goes against the objective of securing transmission. A digital version transmitted securely, directly from the physician to the pharmacist, would probably decrease the odds of theft and falsification.
The lack of digitalisation of counterfoil prescriptions prevents stolen prescriptions from being quickly blocked. Currently, some time elapses between when the physician realises and reports the theft and the moment when pharmacies can access the online list of stolen or lost prescriptions. In addition, pharmacies are supposed to manually check whether the counterfoil prescription received is part of a stolen or lost lot. With a digital system, the whole process would be much faster.
The federal system for stolen and lost counterfoil prescriptions exists in parallel with other cantonal reporting systems that cover ordinary prescriptions. Merging the various systems would make sense.
Counterfoil prescriptions are time-consuming and incompatible with medical data management systems
Cantonal authorities must send pads to physicians and monitor the counterfoil prescription numbers, a process that takes time: “Vaud reports to send out around 2550 counterfoil prescription pads per year. Assuming it takes about 5 minutes to enter data into their tracking system and put the pad in the post, it would take one full-time employee around five weeks annually just filling counterfoil prescription pads requests”32.
Physicians must send counterfoil prescriptions to the pharmacies, usually by post. They must (or at least are supposed to) keep a copy of the used pad and their blue foils for at least 10 years33. Counterfoil prescriptions do not exist online and they are not linked with the patient’s electronic file (DEP or dossier électronique du patient). In practice, physicians often send emails or faxes to pharmacists with controlled medicine prescriptions; the pharmacist will honour the prescription based on this email or fax, provided that the physician promises to send the actual documents by post. Such practices suggest that the paper counterfoil prescription is no longer adapted to modern communication tools.
Pharmacies must keep the white foils. At the pharmacist (or propharmacist physician) level, counterfoil prescriptions are part of a separate paper-based bookkeeping system. In particular, pharmacies must keep a separate sheet of paper for each schedule a or d substance, mentioning the quantities entered and delivered for each separate patient. Again, the process is time-consuming and seems unnecessarily complicated compared to a digitalised system.
Conclusion, recommendations and alternatives
In summary, counterfoil prescriptions are used as a means of communication between physicians and pharmacists, but the information is not used beyond that. In other words, the counterfoil prescription data are not exploited by the physician, the pharmacists, the authorities or the reimbursing insurance company.
In addition, the usefulness of counterfoil prescriptions to limit the volume of controlled medicine prescriptions is very relative. Apart from the psychological effect of having to complete a specific document, due to a lack of time and resources, nobody really controls counterfoil prescriptions. Moreover, the very idea that a cumbersome bureaucratic procedure should serve to discourage the prescription of certain drugs seems dubious since it is more likely to hinder professionals from doing their job properly. To combat inappropriate prescribing of controlled medicines, appropriate and regular training of physicians and pharmacists seems to us a more effective approach.
In addition to their time-consuming nature, counterfoil prescriptions are not suitable for today’s secure protocols. We recommend that they be abandoned and replaced by electronic secure prescribing. For instance, the HIN Sign34 system allows physicians to write digital prescriptions and pharmacies to validate them automatically with a QR code. Secured emails could also be used. If a secure email is accepted as sufficient to transmit sensitive health data between health professionals, then why not prescriptions? Finally, controlled medicine prescriptions could be integrated into the electronic patient file, which is currently being implemented and will be the subject of a consultation procedure on its compulsory nature in the summer of 202335.
Digital prescribing is already done in inpatient settings. Patients hospitalised in palliative care typically receive the necessary medications, without a counterfoil prescription ever being used. In no way does such a practice prevent the strict control of the medication delivered by the hospital pharmacy to the unit and then to each patient. In fact, it favours security and traceability.
Digital prescriptions would improve research on controlled medicine prescription, patient follow-up, bookkeeping and communication between doctors and pharmacies. It would allow one to verify that the physician actually issued the prescription, as opposed to a theft or a falsification.
To implement our recommendation, amending the NarcA is not necessary because counterfoil prescriptions are not required therein. Amendments to the OCStup may not even be necessary since the form of counterfoil prescriptions is not precisely described even in this ordinance. In our opinion, at some point, the requirement to use digital prescriptions could extend to all prescribed medicines (as opposed to just controlled medicines). Therefore, it could be included in the Therapeutic Products Act only36. From this point of view, no justification would remain for maintaining a specific regime for controlled medicine prescription in the narcotic regulation.
This article is part of an SNF-funded project on the regulation of narcotic medicines (project no. 182477); https://wp.unil.ch/medicaments-sous-controle/.
Footnotes
1 Art. 2 let. g Ordonnance sur le contrôle des stupéfiants (OCStup; RS 812.121.1).
2 Art. 2 NarcA; 2 let. h OCStup.
3 Art. 3 al. 2 let. a et d; 46 al. 2 OCStup. The detailed schedules can be found in the annexes of the Ordonnance sur les tableaux des stupéfiants (OTStup-DFI; RS 812.121.11). Tramadol (synthetic opioid) and zopiclone (z-drugs) are not scheduled as controlled medicines.
5 Counterfoil prescription use is recommended in the 1961 Single Convention on Narcotic Drugs (art. 30 al. 2 let. b no. 2: “If the Parties deem these measures necessary or desirable, [the Parties shall also] require that prescriptions for drugs in Schedule I should be written on official forms to be issued in the form of counterfoil books by the competent governmental authorities or by authorised professional associations”). In Switzerland, “official prescription forms” provided by the Confederation are first mentioned in 1984 in federal law (art. 39 al. 1 OStup of the 18 January 1984). They may have existed before this date without a legal basis, but despite our efforts, ascertaining the exact counterfoil prescription start date was not possible.
6 In Germany, the requirement to use a counterfoil prescription is found in §8 of the Betäubungsmittel-Verschreibungsverordnung (BtMVV). In France, CPFs were introduced in 1948 (art. 49 du Décret du 19 novembre 1948) but abolished as of 1 January 1999 and replaced by secure prescriptions. Ministère de l’emploi et de la solidarité, Circulaire DGS/DH N°98/586, 22 septembre 1998; Article R5132-5 du Code de la santé publique.
7 For instance, in the United Kingdom, see the National Health Service page “Controlled drugs in the Electronic Prescription Service”.
8 We also conducted (either face-to-face or online) interviews with nearly all Cantonal Physicians and Cantonal Pharmacists. We asked them which use they made of counterfoil prescriptions.
9 RS 812.121.
10 Ordonnance sur le contrôle des stupéfiants (RS 812.121.1).
11 Also referred to in this ordinance as “ordonnance de stupéfiants”.
12 In theory, physicians could also use counterfoil prescriptions for other controlled medicines, but they usually avoid doing so, given the costs and constraints.
13 Art. 50 OCStup.
14 Art. 66 ordonnance du 5 décembre 2006 sur les pharmacies, les produits thérapeutiques et les stupéfiants (RS-JU 812.41).
15 Interview with Jura’s Cantonal Pharmacist.
16 Prescription: art. 10 NarcA; use and dispensation: art. 9 et 13 NarcA; 51 al. 2 OCStup.
17 From 1996 to 2011, the OStup (art. 43 al. 2) did not require counterfoil prescriptions for human inpatient care; it was planned to remain as such in the 38 al. 2 of the 2010 OCStup draft. However, the exception to the counterfoil requirement for inpatient care was deleted without explanation in the version of the OCStup that came into force in 2011.
18 Art. 4 al. 1 let. k LPTh.
19 Swissmedic, “New forms for prescription of narcotics by medical professionals”, press release 8 November 2017.
20 The physician decides whether to send the prescription by post or hand it to the patient, depending on the perceived risk of copying or falsification by the patient. Sending it by post takes more time and is more complicated. For dependence traitements (OAT), the canton of Vaud, in its directives, states: “Les ordonnances à souche ou ordinaires ne sont pas remises aux patients mais envoyées par la poste à la pharmacie qui se charge de remettre le traitement, afin d’éviter les utilisations abusives (photocopies), le rajout de médicaments, etc.” Directives du Médecin cantonal concernant la prescription, la dispensation et l’administration des médicaments soumis à la législation sur les stupéfiants destinés à la prise en charge de personnes présentant un syndrome de dépendance, 10 November 2021, 12.
21 In Geneva, for instance, even if propharmacy is prohibited, physicians can order methadone at a pharmacy and deliver it to their patients in their office. The methadone is, in this case, sold by the pharmacy, not by the physician.
22 Ordonnance sur les médicaments du 21 septembre 2018 (OMéd; RS 812.212.21).
[23] Schlegel A, Dommer Schwaller J. Les questions juridiques à la pharmacie. PharmaSuisse. Manuel pratique du pharmacien suisse; 2018. 158–160.
24 Information sent by email by Swissmedic on 27 September 2022.
25 See “Gesperrte Betäubungsmittelrezeptformulare” on Swissmedic’s website.
26 Art. 8 al. 2 of the Règlement d’application de la loi sur les stupéfiants du 26 septembre 2001 (RS-NE 804.30); art. 20 al. 3 of the Règlement sur les produits thérapeutiques, les pharmacies et les drogueries du 18 octobre 2006 (RS-NE 804.10).
27 Art. 31 al. 2 let. k of the Ordonnance sur les pharmacies, les produits thérapeutiques et les stupéfiants du 5 décembre 2006 (RS-JU 812.41).
28 Nicollier L, Du Pasquier S, Berger J, Gouveia A. Prescription des opioïdes en médecine générale pour les douleurs chroniques non cancéreuses. Rev. Med. Suisse. 2022; 8(796): 1761.
29 In Geneva: art. 6A du Règlement relatif à l’application de la loi fédérale sur les stupéfiants et les substances psychotropes (RaLStup ; K 4 20.02). Recently, the Cantonal Pharmacist asked pharmacists to send counterfoil prescriptions for OAT, for control purposes. See Service du Pharmacien cantonal, Envoi des ordonnances à souche relatives aux traitements des dépendances, circulaire du 21 janvier 2021; in Vaud: 17 al. 1 let. e du Règlement sur les stupéfiants du 25 mars 1987 (BLV 812.11.1).
30 Gumy C, Huissoud T, Dubois-Arber F. Prevalence of methylphenidate prescription among school-aged children in a Swiss population: Increase in the number of prescriptions in the Swiss canton of Vaud, from 2002 to 2005, and changes in patient demographics. J. Atten. Disord. 2010;14:267–272; Montandon J-B, Médioni L. Evolution du nombre de prescriptions de Ritaline (méthylphénidate) dans le canton de Neuchâtel entre 1996 et 2000. BAG Bull. 2002;15:284–290.
31 Cf. Swissmedic website: “MESA: Application pour les substances soumises à contrôle”; art. 60 et 61 OCStup.
32 Schmitt-Koopmann C, Baud C-A, Junod, V, Simon O. Switzerland’s narcotics regulation jungle: Off-label use, counterfoil prescriptions, and opioid agonist therapy in the French-speaking cantons. Int. J. Environ. Res. Public Health. 2021; 18 (13164).
33 Art. 62 al. 3 OCStup.
34 https://www.hin.ch/fr/services/hin-sign/ordonnance-electronique-hin.
35 See the law and the ordinance on the electronic patient record (LDEP; ODEP); Federal Office of Public Health, Développement du dossier électronique du patient, press release, 27 January 2023.
36 Art. 51 al. 2 and 4 OMéd already mention digital prescriptions.
Valérie Junod, Professor at the Faculty of Law of the University of Geneva and the Faculty of Business and Economics of the University of Lausanne, Counsel to the law firm JMLP, Geneva
Carole-Anne Baud, Dr. iur and post-doctoral fellow at the Faculty of Business and Economics of the University of Lausanne on the SNF project 100011_182477, caroleanne.baud[at]unil.ch
Caroline Schmitt-Koopmann, MSc in Pharmaceutical Sciences and PhD candidate on the SNF project 100011_182477
Jean-Christophe Devaud, Dr. sc., hospital pharmacist FPH, head of the pharmaceutical logistics unit, Lausanne University Hospital (CHUV)
Margaux Bonnard, BLaw, student-assistant at the Faculty of Business and Economics of the University of Lausanne
Sophie Pautex, professor at the Faculty of Medicine of the University of Geneva and head of the Palliative Medicine Service of the Geneva University Hospital (HUG)
Barbara Broers, professor at the Faculty of Medicine of the University of Geneva and head of the Addiction Unit of the Geneva University Hospital (HUG)
Olivier Simon, Senior physician at the Addiction Medicine Service of the Lausanne University Hospital (CHUV), lecturer and researcher at the Faculty of Biology and Medicine of the University of Lausanne
