The treatment of iron deficiency without anaemia (in otherwise healthy persons)
DOI: https://doi.org/10.4414/smw.2017.14434
Sportmedizinisches Zentrum Ittigen bei Bern, Ittigen,
The treatment of iron deficiency without anaemia (in otherwise healthy persons)
Summary
Iron deficiency is the most widespread and frequent nutritional disorder in the world. It affects a high proportion of children and women in developing countries and is also significantly prevalent in the industrialised world, with a clear predominance in adolescents and menstruating females. Iron is essential for optimal cognitive function and physical performance, not only as a binding site of oxygen but also as a critical constituent of many enzymes. Therefore iron deficiency at all levels – nonanaemic iron deficiency, iron deficiency with microcytosis or hypochromia and iron deficiency anaemia – should be treated. In the presence of normal stores, however, preventative iron administration is inefficient, has side effects and seems to be harmful.
In symptomatic patients with fatigue or in a population at risk for iron deficiency (adolescence, heavy or prolonged menstruation, high performance sport, vegetarian or vegan diet, eating disorder, underweight), a baseline set of blood tests including haemoglobin concentration, haematocrit, mean cellular volume, mean cellular haemoglobin, percentage of hypochromic erythrocytes and serum ferritin levels are important to monitor iron deficiency. To avoid false negative results (high ferritin levels in spite of iron deficiency), an acute phase reaction should be excluded by history and measurement of C-reactive protein. An algorithm leads through this diagnostic process and the decision making for a possible treatment. For healthy males and females aged >15 years, a ferritin cut-off of 30 µg/l is appropriate. For children from 6–12 years and younger adolescents from 12–15 years, cut-offs of 15 and 20 µg/l, respectively, are recommended.
As a first step in treatment, counselling and oral iron therapy are usually combined. Integrating haem and free iron regularly into the diet, looking for enhancers and avoiding inhibitors of iron uptake is beneficial. In order to prevent reduced compliance, mainly as a result of gastrointestinal side effects of oral treatment, the use of preparations with reasonable but not excessive elemental iron content (28–50 mg) seems appropriate. Only in exceptional cases will an intravenous injection be necessary (e.g., concomitant disease needing urgent treatment, repeated failure of first-step therapy).To measure the success of treatment, the basic blood tests should be repeated after 8 to 10 weeks. Patients with repeatedly low ferritin will benefit from intermittent oral substitution to preserve iron stores and from long term follow-up, with the basic blood tests repeated every 6 or 12 months to monitor iron stores. Long-term daily oral or intravenous iron supplementation in the presence of normal or even high ferritin values is, however, not recommended and is potentially harmful.
Introduction
Iron deficiency is the most common and widespread deficit globally, with a clear predominance in adolescents and in menstruating women [1]. It greatly affects developing countries, where it is the major cause of anaemia [2]. The WHO claims that 40–50% of these anaemic conditions are iron deficiency anaemia (IDA), and therefore preventable and treatable [3]. In the industrialised world, as iron deficiency is one of the few nutritional deficits, its prevalence is significant.
Data from Australia [4], Denmark [5–7], Switzerland [8, 9] and the US [10, 11] provide the data on prevalence. In childhood, both sexes show similar a prevalence from 3–9%. In adolescence, with ongoing growth in puberty and the onset of menarche, iron deficiency is more frequent in females at 11–33% compared with 3.5–13% in males; this figure gradually and distinctly declines in post-adolescent men. During childbearing age the prevalence in women remains quite high (range 9–22%) compared with men of the same age-group (1–2% only). After the menopause, ferritin values in women rise with a lowering of the prevalence of iron deficiency to 2.3–7%, which comes closer to the values of their male counterparts with a range of 1.4–4%.
In order to develop the topic of iron deficiency, this article discusses the general function and metabolism of iron, the influence of iron on performance and cognition, basic measurements to diagnose iron deficiency, the stages of iron deficiency and, finally, treatment.
General function of iron and iron metabolism in the human body
Iron is a transition metal and has multiple functions in more than 180 biochemical reactions in the human body, including electron transport in redox reactions (cytochromes, sulphuric proteins), redox catalytic functions (cytochrome p450, catalase, peroxidase) and reversible storage and transport of O2 (haemoglobin, myoglobin). It also plays an important role in the production of neurotransmitters, and is essential in synaptogenesis and myelinisation. Oxidative phosphorylation is the most critical biochemical pathway in which iron is involved [12, 13].
The total body content of iron amounts to approximately 4 g in men and 2.5 g in women. This iron is divided between three active sites: haemoglobin 65%, myoglobin 10% and enzymes 5%. The rest (20% of the total) remains as inactive, depot iron in the form of ferritin and haemosiderin. Finally, 0.2% of the total iron exists as transport iron in the form of transferrin. In adolescents, the relative amount of iron in the different compartments is comparable but may vary slightly depending on body size and initiation of menses [12, 14, 15].
The usual loss of iron (1 mg per day in males and 2 mg per day in females) due to gastrointestinal epithelial shedding and menstruation is compensated by absorption in the small intestine [13, 14]. Of the 10–14 mg of iron ingested, the enterocytes absorb only about 0.5–2 mg (5–15%) [16, 17]. Nevertheless, during increased loss (e.g., menstruation, other bleeding, haemolysis) and elevated demand (e.g., growth, pregnancy, high performance sport [18, 19]), adequate uptake is guaranteed through an up to four-fold increase in intestinal absorption, as long as sufficient iron is provided by nutritional intake [14, 17].
The absorbed iron is stored in ferritin in the cytoplasm of the enterocytes. For export to the plasma, iron is carried out by ferroportin on the basolateral surface of the enterocytes [12]. There, iron is bound to transferrin and transported to the liver, where it is stored as ferritin or transferred to iron-consuming tissues such as bone marrow.
Ferroportin is important in the tight regulation of iron homeostasis. The main regulatory molecules are hepcidin and erythroferrone [16, 20, 21]. Hepcidin, synthesised in hepatocytes, regulates iron export out of the storing cells. In phases of high iron loading and in response to inflammatory processes, the synthesis of hepcidin is increased, leading to the internalisation of ferroportin on enterocytes, which in turn blocks iron transportation into the circulation. The same mechanism leads to a blockade of iron within the macrophage system, thus preventing the transfer of iron from macrophages to erythroblasts, the precursors of erythrocytes [12, 20] (fig. 1). Hepcidin synthesis is suppressed by erythropoietic activity and anaemia: hypoxia induces erythropoietin production in the kidneys, erythropoietin stimulates the production of erythroferrone in erythroblasts and erythroferrone blocks the production of hepcidin [21]. This allows increased intestinal absorption and utilisation of iron from the macrophages and enterocytes under conditions of elevated iron loss or increased demand [16]. Newer results show that not only iron loading and inflammation, but also intensive exercise induce hepcidin bursts causing a blockage of iron [16].
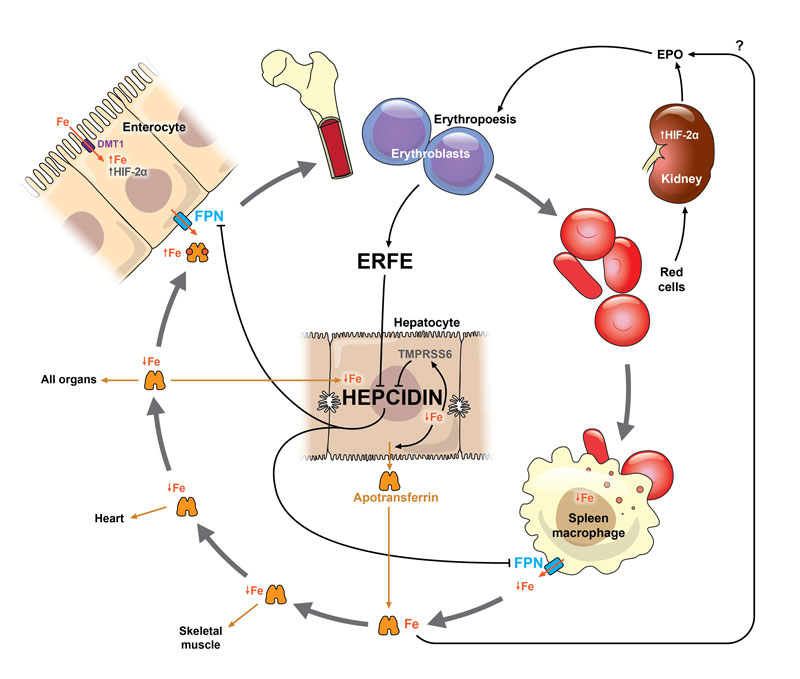
Figure 1 Iron cycle and mechanisms of adaptation to iron deficiency, adapted from Camaschella 2015 [22] and Camaschella et al. 2016 [20].
Tissue hypoxia leads to production of hypoxia inducible factor 2α (HIF-2α). As a consequence the kidneys produce erythropoietin (EPO), which leads to an enhancement of erythropoiesis, and hypochromic microcytic red cells are produced owing to a low availability of iron. EPO is also influenced in part by body iron via an as yet only partially understood mechanism [16, 20]. In enterocytes HIF-2α increases the expression of divalent metal transporter (DMT 1) on their apical surface and the uptake of dietary iron will be increased. The increased erythropoiesis suppresses the production of hepcidin through the intermediate erythroferrone (ERFE), which is produced in erythroblasts. Hepcidin levels are further depressed in response to a reduction in the usual signals that maintain its production: low iron content of the liver, increase in inhibitor transmembrane protease, serine 6 (TMPRSS6). Ferroportin (FPN) is no longer degraded because of the low hepcidin levels and it exports the available iron across the basal membrane of the enterocytes and from macrophage stores to enter circulation. As long as stores are exhausted and no therapy started, iron availability remains low and consequently the uptake of iron by all cells and organs (e.g., heart, skeletal muscle) is reduced.
Influence of iron on physical performance and cognition
Iron is, in addition to its function in oxygen transport, a key component of the enzymatic system of the respiratory chain. These presumably distinct roles were investigated in early animal studies [23, 24]. In these studies, the investigators aimed at differentiating between a decline in performance due to anaemia and a decline due to enzymatic impairment. With an animal model in a crossover experiment of iron depletion and crossover transfusion to correct anaemia, it was shown that not only anaemia but also iron depletion without anaemia led to a significantly decreased number of mitochondria and reduced activity of the respiratory enzymes. The authors therefore postulated that iron depletion without anaemia affected oxidative capacity, whereas anaemia affected mostly oxygen transport [23, 24].
At present the cut-off level of ferritin is still debated, with values ranging from 15 µg/l from the World Health Organization (WHO) [2] to 16–32 µg/l [25, 26] in studies that used the “gold standard procedure of bone marrow staining”. It has to be noted that studies investigating this matter were not all conclusive and some had controversial results [26, 27].
If not only erythropoiesis, but also clinical symptoms of an iron deficiency such as fatigue (but not yet performance) are considered, the cut-off may be slightly higher. Recently Krayenbuehl et al., in their double-blind randomised study of intravenous iron administration to nonanaemic premenopausal women with low ferritin and fatigue, showed an improvement in mood state in the group with ferritin <15 µg/l [28]. Two studies looked at fatigue and mood state in premenopausal nonanaemic women receiving oral supplementation from a family doctor [29, 30]. Their proposed cut-off of 50 µg/l must be interpreted with caution because of methodological concerns (e.g., stool colouration by iron was not blinded, definition of iron deficiency was based on a limited number of variables). Finally, there are a few studies that included a performance measurement offering more objective data (table 1).
Table 1 Randomised blinded interventional trials investigating nonanaemic iron deficiency and performance.
|
First author
|
Performance measurement
|
No.
Study population
|
Inclusion criteria
|
Intervention
|
Results
|
Conclusion
|
| Burden 2014 [31] |
VO2max
Time to exhaustion
Running economy
Haemoglobin mass |
15
runners, 9F
VO2max 64.5 ml/kg·min
6 min VO2max
76.7 ml/kg·min |
F: Hb >120 g/l, Fer <30 μg/l
M: Hb >120 g/l, Fer <40 μg/l |
IV 500 mg Fe carboxymaltose vs placebo
Testing at BL, after 7 d and after 4 w
Blood sampling as above and 1 day after injection |
– Fer↑, traSat↑, serum iron↑ in Tx, Fer→ in Pl
– Hepcidin↑ from day 1 up to 4 w in Tx
– Hb mass→, VO2max→, running economy→, time to exhaustion→ in Tx and Pl |
Despite IV iron in abundance no improvement in Hb mass and performance in NAID male (Fer <40 μg/l) and female Fer <30 μg/l runners.
Distinct rise in hepcidin for at least 4 weeks. |
| Garvican 2014 [32] |
Treadmill running with VO2max, time to exhaustion
Hb mass |
27
highly trained distance runners
13 M, 14 F
With (low group, LG) or without (control group, CG) low iron status |
LG: Fer <35 µg/l; trSat <20% or Fer <15 μg/l
CG: Fer <65 μg/l |
Oral: 105 g elemental iron; 2×/d in LG, 1×/d in CG
vs
IV: 2–4 injections Fe-carboxymaltose based on iron status (mean IV dose CG 375 mg, mean IV dose LG 550 mg)
Testing at BL and after 6 w |
– Fer↑ with oral and IV treatment, significantly↑ with IV
– Hb→ in all groups
– In LG IV: Hb mass↑, VO2max↑and time to exhaustion↑
In CG subgroup with “suboptimal” iron status, no change in Hb mass, VO2max and time to exhaustion. |
IV iron improves Hb mass and performance only in iron deficient male and female runners (Fer <35 ug/l and trSat <20% or Fer <15 μg/l).
No change with oral treatment, no change with ferritin <65 µg/l. |
| Della-Valle 2014 [33] |
4 km time trial
VO2peak |
40
female rowers
at the beginning of the season |
Hb > 120g/l, Fer <20 μg/l |
Oral FeSO4 100 mg/d vs placebo for 6 w
Testing at BL and after 6 w of training |
– fat free mass↑ and VO2peak↑ in Tx and Pl
– Fer↑ in Tx
– energy expenditure in Tx↑
– lactate response↓ in first half of time trial in Tx, and ↓ 5 min after time trial |
6 weeks of oral iron improves rowing economy in NAID female rowers (Fer <20 μg/l) at the beginning of the season.
The placebo group shows a similar improvement in VO2max, however. |
| Waldvogel 2012 [34] |
Chester step test (r = 0.92 to VO2max)
Fatigue (VAS 10) |
154
female blood donors |
Hb >120 g/l, Fer ≤30 μg/l |
Oral FeSO4 80 mg/d vs placebo for 4 w
Testing at BL (1 w after donation of 450 ml blood) and after 4 w. |
In Tx Hb↑, Fer↑ compared with Pl
No significant effect for fatigue, aerobic capacity (step test), mood disorder, quality of life |
4 weeks of oral iron shows no performance improvement, no improvement of fatigue or mood in female blood donors.
Limitation: substitution not long enough. |
| McClung 2009 [35] |
2 mile running time
Profile of mood state (POMS) |
219
female soldiers during basic combat training
Three groups:
IDA (Pl 17, Tx 18)
ID (Pl 14, Tx 14)
normal (Pl 51, Tx 52) |
No inclusion criteria.
IDA defined as
Hb <120 g/l and ≥2 of: Fer <12 μg/l, traSat <16%, RDW >15%
ID defined as ≥2 of iron crit. |
Oral FeSO4 100 mg/d vs Pl
Testing at BL and after 8 w of basic combat training |
– RDW↑, sTfr↑, Fer↓ in Tx and Pl.
– decrement in iron status ↓ in Tx
– with IDA, 2-mile running time↓ in Tx; but not in Pl, or in normal.
– POMS↑ in Tx, Pl and normal; only in IDA vigour scores of POMS↑ |
8 weeks of oral iron improve performance in NAID female military recruits. |
| Hinton 2007 [36] |
VO2max
60 min submax. cycle ergometer test (at 60% VO2max) |
20
recreationally trained (3M, 17F) |
Hb >120g/l (F) or >130g/l (M), Fer <16 μg/l, sTfr >8 mg/l or sTfr/log Fer index >4.5 |
Oral 30 mg/d elemental iron as FeSO4 vs placebo
Testing at BL and after 6 w |
– Fer↑ in Tx, Hb and haematocrit →
– in Pl ventilatory threshold↓, in Tx ventilatory threshold→
– energetic efficiency during submaximal test ↑ in Tx |
6 weeks of oral iron maintain performance and improve submaximal energetic efficiency in recreationally trained men and women (Fer <16 μg/l, sTfr >8mg/l, or sTfr/log Fer index >4,5).
compared with a decrease in the placebo group. |
| Brownlie 2004 [37] |
15 km time trial on cycle ergometer
VO2max |
41
untrained women
Training of 30 min/d, 5× for the final 4 w |
Hb >120 g/dl, Fer <16 μg/l |
Oral FeSO4 100 mg/d vs placebo
Testing at BL and after 6 w |
Time in time trial↓, percentage of VO2max↓, work rate↓ in Tx when sTfr >8 mg/l
No difference with normal sTfr |
6 weeks of oral iron improve performance in the subgroup of nonanaemic iron deficient untrained women (Fer <16 μg/l) with elevated sTfr >8 mg/l. |
Since the randomised controlled trial of Bruner et al. [38] in 1996, which looked at US female adolescents with nonanaemic iron deficiency (NAID) and which showed an improvement in verbal learning and memory with oral iron, there is increasing evidence that not only IDA but also NAID affect cognition. Falkingham et al. found, in their 2010 systematic review and meta-analysis, evidence that iron supplementation improved attention and concentration in adolescents and women with IDA and NAID, regardless of baseline iron status [39]. Since then one randomised controlled trial [40], two other systematic reviews [41, 42] and a pilot study [43] supporting these findings have been published. All the authors pointed out that further well-designed, blinded and independently funded studies of at least 1 year duration, looking at different age groups and varying levels of baseline iron status, and using validated tests of cognition are needed to confirm and extend these results. Table 2 gives an overview of randomised controlled clinical trials relevant to iron deficiency and cognitive function.
Table 2 Randomised blinded interventional trials investigating nonanaemic iron deficiency and cognition.
|
First author
|
Cognition measurement
|
No.
Study population
|
Inclusion criteria
|
Intervention
|
Results
|
Conclusion
|
Baum-gartner 2012
[40] |
Hopkin’s verbal learning test (HVLT) and subscales of Kaufman assessment battery for children (KABC) |
321
South African school children, age 6–11 y
79 Fe + DHE/EPA
81 Fe + Pl
81 Pl + DHE/EPA
80 Pl + Pl |
Hb >80 g/l and ID defined as either Fer <20 μg/l or ZnPP >70μmol/mol haem or sTfr >8.3 mg/l.
In all four groups about 20% were anaemic (Hb <115 g/l) |
2×2 factorial trial:
(1) 50 mg FeSO4 + 420 mg DHA / 80 mg EPA
(2) Fe + Pl
(3) Pl + DHA/EPA
(4) Pl + Pl
All 4×/w for 8.5 mo |
– with Fe-Tx cognition↑ (↑number of words HVLT) vs Pl.
– in children with NAID, HVLT→ and KABC→
– in all groups KABC↑ for learning abilities, whereas sequential processing↓
– Fer↑ in (1) 21.4–58.4 μg/l and (2) 20.0–62.7 μg/l
– Hb→ in all 4 groups |
Several months of oral iron improve cognition in iron-deficient South African school children(Fer<20μg/l, or ZnPP>70μmol/mol haem or
sTfr>8.3mg/l)
Limitations: 20% of the children were anaemic. The complex study design makes it difficult to attribute the observed effects. |
Murray-Kolb 2007
[44] |
Cognitive abilities-attention, cognitive abilities-memory, cognitive abilities learning, Shipley Inst Scale (IQ) |
149
USA women, age 18–35 y
Control-Pl 21
Control-Fe 21
ID-Pl 37
ID-Fe 36
IDA-Pl 15
IDA-Fe 19 |
Control:
Hb >120 g/l, Fe status normal (mean Fer 45 ± 20 μg/l)
ID: Hb >120 g/l, 2 abFeSt (mean Fer 8.9 ± 4 μg/l)
IDA: Hb 105–119 g/l and 2 abFeSt |
Oral elemental iron 60 mg/d
For 16 w |
– Tx: Fer↑ with 5- to 7-fold ↑ in cognitive performance, ↑ in Hb related ↑speed in completing cognitive tasks.
– Fer↑ in Cn-Fe, ID-Fe, IDA-Fe (mean change 20/16/15μg/l) and ID-Pl (mean change 7μg/) but not in Cn-Pl and IDA-Pl
-Hb↑ in IDA-Fe (mean change 12g/l) and in IDA-Pl (mean change 7g/l) but → in Cn-Pl, Cn-Fe, ID-Pl, ID-Fe |
16 weeks of oral iron improve cognitive performance in NAID US women (mean Fer 8.9 ± 4 μg/l) |
Lambert 2002
[45] |
Visual search and attention (AC), Hopkin’s verbal learning (HVLT), Stroop task, reading span |
116
New Zealand female high school students age 12.5–17.9 y
Fe 57
Pl 59 |
Hb >120 g/l
Fer <12 μg/l |
Oral elemental Fe 105 mg/d vs placebo
For 8 w |
– In Tx, immediate word recall in HVLT↑, recall of recent words↑ and reading span↑; in Pl all →
- Fer↑ in Tx (mean change 17 μg/l), in Pl → |
8 weeks of oral iron improves cognitive performance
in NAID NZ female high-school students (Fer <12 μg/l). |
Lynn-Harland 1998
[46] |
Raven’s Color Progressive Matrices (IQ) |
415
England teenagers at 7 comprehensive schools, age 12–16 y
Fe 208
Pl 205 |
ID:
Fer ≤12 μg/l but any Hb
Control: the others |
Oral elemental Fe 80mg/d
16 w
BL (1 w after donation of 450 ml blood) and after 4 w. |
In ID, ↑ in IQ with a gain of 5.8 IQ points compared with Pl. |
16 weeks of oral iron improves IQ in iron deficient English teenagers (Fer ≤12 μg/l)
Limitation: no clear inclusion criteria for haemoglobin. |
Bruner 1996
[38] |
Visual search and attention, Hopkin’s verbal learning test, digit symbol modalities, attention |
81
USA adolescent girls, mean age 16.2 y and 15.7 y
Fe 40
Pl 41 |
African American Hb >115 g/l, white American Hb >120 g/l
Fer <12 μg/l for all |
Oral elemental iron 260 mg/d
For 8 w |
– In Fe-Tx, ↑ in test of verbal learning and memory compared with Pl (p <0.02)
– Fer ↑ in Tx (27.3 vs 12.1 μg/l, p <0.001) |
8 weeks of oral iron improves verbal learning and memory in NAID US adolescent girls (Fer <12 μg/l). |
Groner 1986
[47] |
Vocab (IQ), digit symbol, arithmetic, consonant trigram, Rey auditory verbal learning, digit span |
38
USA pregnant women, age 14–24 y
Vitamins with Fe 19
Pl (vitamins alone) 19 |
Hb >120 g/l
Fer <40–60 μg/l |
Oral elemental iron 60 mg/d
For 4 w |
– In Fe-Tx, ↑ in most sensitive measure of short-term memory and three subtests.
– Hb↑ in Tx |
4 weeks of oral iron improves short term memory in nonanaemic pregnant women with ferritin <40–60 μg/l. |
Based on the cited results, overviews on the influence of iron deficiency on performance and cognition, and according to several review articles, a ferritin cut-off of 30 µg/l in adults seems to be the most plausible [48–55]. According to the available data, iron treatment of otherwise healthy persons with ferritin levels above this value does not bring any benefit, for neither performance [31, 32] nor cognition [40, 44].
Basic measurements to diagnose iron deficiency
The basic measurements needed to diagnose iron deficiency are shown in table 3: these are haemoglobin, haematocrit and erythrocyte count, with calculation or measurement of the red cell indices mean corpuscular volume (MCV) and mean corpuscular haemoglobin (MCH), the percentage of hypochromic erythrocytes (%HYPO), ferritin and C-reactive protein. The algorithm presented in figure 2 summarises the diagnostic process.
Table 3 Relevant tests to define anaemia, NAID, IDMH and IDA.
|
Parameter
|
Cut-off
|
Comment/caveat
|
| Hb |
Women: >120 g/l
Men: >140 g/l |
Defines diagnosis of anaemia. Cut-off values are affected by gender, age, and ethnicity.
WHO definition is >120 g/l for women and >130 g/l for men. |
| MCH |
>28 pg |
Categorises anaemia |
| MCV |
>80 fl |
Categorises anaemia (owing to a certain instability after blood sampling, less useful than MCH) |
| %HYPO Percentage of hypochromic erythrocytes [56–58] |
%HYPO <10 |
Indicates an iron deficiency and its influence on haematopoiesis at an earlier stage, particularly IDMH. Iron deficiency affecting erythropoiesis is followed by an increase in %HYPO within 1–2 weeks. In patients with low ferritin (meaning empty iron stores), a normal %HYPO indicates that erythropoiesis is not yet affected.
Limitation: availability of the method, careful interpretation needed in cases of possible thalassaemia, as in these patients %HYPO rises with normal iron stores [59]. |
| Reticulocyte count, absolute |
20–100 × 109/l
Reference values of the manufacturer need to be respected. |
Assesses red blood cell production and helps to further classify an anaemia (hypo- and hyper-regenerative) |
| Reticulocyte indices [60–63] |
MCVr 92–120 fl
(mean cellular volume of reticulocytes)
CHr 28–35 pg [60]
(amount of Hb in reticulocytes, measured in Advia 120)
Ret-He 28–35 pg [64]
(amount of Hb in reticulocytes, measured in Sysmex NE 2100) |
A lowering of the MCVr and, even more specifically, the CHr or Ret-He are very early indicators of the iron demand of erythropoesis. A value <28 pg is equivalent to a functional iron deficiency. CHr or Ret-He react quickly, within 48–72 h, to an increased demand or lowered supply, as compared with e.g. MCV and MCH which react only within weeks.
Limitation: availability of the method |
| Ferritin |
30 μg/l |
The most widely used parameter for IDA.
Limitations: as an acute phase protein, ferritin is increased during inflammation and infection, after intensive exercise, in pregnancy and with liver damage, see also CRP. |
| CRP |
<3 mg/l |
Acute phase protein, indicating infection and inflammation |
| Free serum iron |
– |
Obsolete, as not representative of the amount of body iron. Only to be used for acute iron intoxication and to calculate transferrin saturation.
Limitations: daytime and interindividual variability. In the morning, values are showing a peak being more than twice as high as values measured 12 hours later. Lowered in acute phase reactions and elevated in haemolysis after blood sampling. |
| Transferrin saturation |
>20% |
<20% indicates iron deficiency
Limitations: acute phase reactions lower the transferrin saturation without iron deficiency. Moreover, as free serum iron is used for the calculation transferrin saturation may also vary [65]. |
| sTfR |
Reference values of the manufacturer need to be respected, as they differ substantially.
Woman: 0.75–1.5 mg/l
Men: 0.75–1.75 mg/l |
Indirect marker to define IDMH and NAID. Similar sensitivity to ZnPP for NAID and IDMH. Not influenced by inflammation and exercise [52].
Limitation: mostly elevated during erythropoiesis, so may only be of additional value. |
| ZnPP [66, 67] |
<50 μmol/mol Hb excludes iron deficient erythropoiesis,
>100 μmol/mol Hb indicates iron deficient erythropoiesis |
Indirect marker to define IDMH and NAID. Early marker of NAID and increases to higher values in IDMH [53].
Not as much influenced by inflammation as ferritin, is entering routine testing and is of additional value for judging iron status of erythropoiesis. |
| Hepcidin |
Not yet a routine laboratory test |
Key regulator of iron absorption from erythrocytes [20]
Elevation impairs haematopoiesis [16, 68] |
| CRP = C-reactive protein; Hb = haemoglobin; IDA = iron deficiency anaemia; IDMA = iron deficiency with microcytosis or hypochromia; MCH = mean cellular haemoglobin; MCV = mean cellular volume; NAID = nonanaemic iron deficiency; sTfR = soluble transferrin receptor; ZnPP = zinc protoporphyrin |
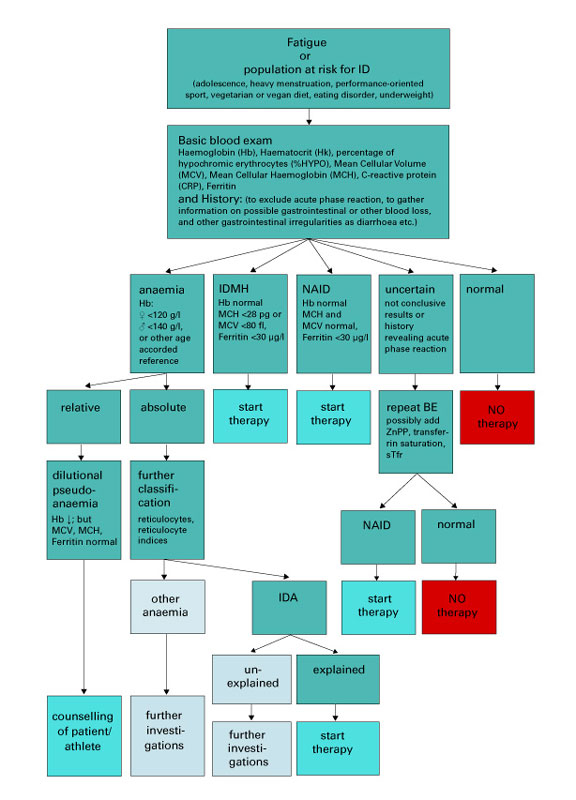
Figure 2 Algorithm for diagnosis and treatment of iron deficiency (in otherwise healthy adults).
BE = basic blood examination; IDA = iron deficiency with anaemia; IDMH = iron deficiency with microcytosis and/or hypochromia; NAID = nonanaemic iron deficiency.
Relative, dilutional pseudoanaemia is explained in reference [48].
Stages of iron deficiency
Figure 3 depicts the stages in the continuum of iron deficiency, in accordance with the proposition in the recent consensus paper of the Swiss Society of Sports Medicine on iron deficiency [48]. When iron losses exceed absorption or absorption falls below demand, initially iron stores will deplete, resulting in a reduced ferritin level. At a certain point, the stored iron is too low to provide the tissues with sufficient iron. This will induce the production of zinc protoporphyrin and an increase of soluble transferrin receptor (sTfR). As at this point haemoglobin, MCV and MCH are still normal; this condition is called nonanaemic iron deficiency (NAID). NAID is defined as a deficiency of iron without affecting haematopoiesis.
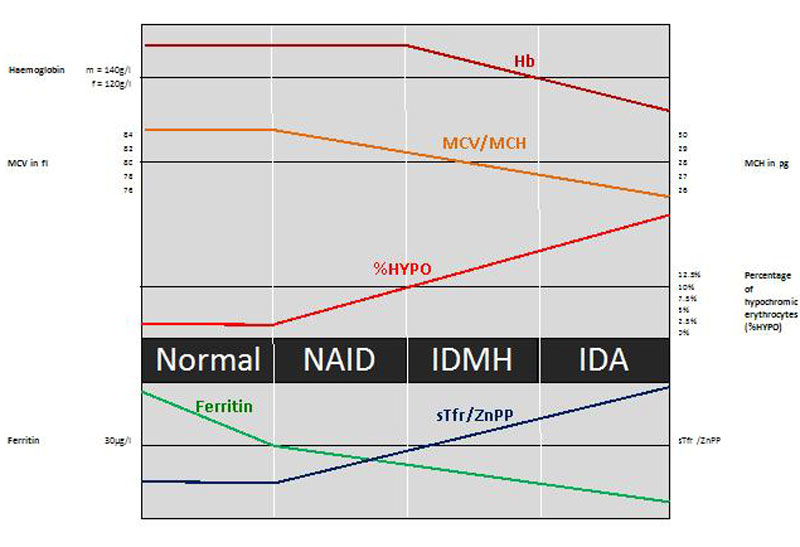
Figure 3 Stages of iron deficiency.
%HYPO = percentage of hypochromic erythrocytes; IDA = iron deficiency anaemia; IDMH = iron deficiency with microcytosis and/or hypochromia; MCH = mean cellular haemoglobin; MCV = mean cellular volume; NAID = nonanaemic iron deficiency; sTfr = soluble transferrin receptor; ZnPP = zinc protoporphyrin
If iron balance remains negative, the youngest red cells will be insufficiently haemoglobinised and thus appear hypochromic and microcytic, with first a rise in the percentage of hypochromic erythrocytes (%HYPO) and a slight but possibly not yet visible reduction in the MCH and MCV of the entire cell population [57, 58]. If iron deficiency continues, MCH and MCV will drop below the lower limit of the normal ranges (28 pg and 80 fl, respectively) and iron deficiency with microcytosis and/or hypochromia (IDMH) develops. IDMH is defined as an iron deficiency affecting haematopoiesis. In this case, ferritin is <30 µg/l, %HYPO is above 10%, the red cell indices are quite often, but not always, affected and the concentration of haemoglobin is still normal (men >140 g/l, women >120 g/l: haemoglobin limits defining an anaemia for middle-aged persons originating from Western European countries) [69].
Ultimately, haemoglobin concentrations will drop below the lower limit of the normal range and frank IDA is established. In IDA, ferritin and haemoglobin are lowered and the red cell indices are reduced or normal [69]. In the diagnostic process it is important to understand where the iron deficiency comes from. In addition to identifying populations at risk, the medical history needs to focus on possible blood loss (mainly gastrointestinal, possibly relapsing nose bleeding), nutritional deficits and other gastrointestinal irregularities, the latter possibly revealing a problem with iron uptake, such as coeliac disease, an important differential diagnosis in a case of relapsing iron deficiency. In cases of unexplained IDA, an extensive diagnostic work-up is indicated.
Another interesting situation is the functional iron deficiency encountered in patients with anaemia of chronic disorder or a tumour, or haemodialysis patients. Here, iron demand is higher than the iron supply out of iron stores. In consequence, hypochromic reticulocytes and erythrocytes are formed, the haemoglobin in reticulocytes (CHr or Ret-He,
for explanation see footnote to table 1) falls to <28 pg [60, 61]. This situation is due to elevated interleukin-6 and hepcidin levels seriously impairing iron turnover and may therefore occur with normal or often even elevated ferritin values, reflecting normal iron stores [20, 60].
Treatment of iron deficiency
Nutrition
The first step in the treatment of iron deficiency is correction of the nutritional iron intake. In nutrition, iron is present as haem iron (mainly in meat) and free iron (Fe2+ or Fe3+). Oral uptake studies show that the uptake of haem iron is much better than the uptake of free iron [70]. For the latter, uptake of Fe2+ is better than uptake of Fe3+ [71]. Meat, liver, poultry or fish contain haem iron as well as free iron. A vegetarian diet contains only free iron. The bioavailability of iron is very variable, ranging from 5 to 15%, and greatly depends on iron stores [72]. In the case of iron deficiency, a significant increase in iron bioavailability up to 35% can be observed [73]. Furthermore, iron uptake in the intestinal tract is influenced by various nutritional factors, including both enhancers and inhibitors. Substances enhancing iron uptake are vitamin C, peptides from partially digested muscle tissue, fermented food, and organic acids like malate or citrate. Substances inhibiting iron uptake are phytates, oxalates, polyphenols (in black tea and coffee), peptides from partially digested vegetable proteins and calcium [49, 50]. The nutritional intake should be 14 mg per day [74, 75]. General recommendations for an optimal dietary iron intake in sports include an adequate energy intake, especially for athletes with low body mass index as they suffer more frequently from iron deficiency [18, 19, 76]. Whether catabolism related to low energy intake influences the hepcidin regulation and down-regulates iron uptake remains open to debate. In general, regular consumption of meat, poultry or fish at least five times per week is recommended as they are the main contributors to nutritional iron intake. Complementary eating of wholemeal products and daily legumes and green vegetables is suggested. Furthermore, it is beneficial to replace tea and coffee by a glass of orange or citrus fruit juice with an iron containing meal as vitamin C enhances iron uptake [50, 73]. For vegetarians, the goal is to reach a high load of iron through their vegetable diet. Even if nutrition is important in iron homeostasis, in the human organism an IDA cannot be corrected by nutrition alone, as this would mean eating kilograms of iron-containing products (e.g., liver).
Oral iron
Usually, dietary counselling and oral iron therapy are combined. Oral preparations differ in the amount and type of iron (Fe2+ or Fe3+), their complex-forming substrate and their galenic form. Novel products combine iron with vitamin C. In a dosage-finding study in elderly patients with IDA, Rimon et al. compared three dosages of oral iron: 15, 50 and 150 mg per day. They were able to show that iron supplementation at the level of the recommended daily allowance (RDA: 15 mg of elemental iron) already led to significant increases in the iron status. In these anaemic elderly people, administration of 50 and 150 mg of elemental iron did not show further benefit, but had significantly more side effects, particularly in the highest dose group [77]. As there is further evidence that oral iron loading increases circulating hepcidin [78], the recommended dose of oral iron should not be too high. In a recent comparison of randomised controlled trials of oral iron supplementation that looked at iron status and performance in active women, 100 mg of FeSO4 (approximately 20 mg elemental iron) was shown to be effective [79]. We therefore recommend a supplement of 28 to 50 mg of elemental iron once daily. Oral iron is in general well tolerated and effective [80]. Side effects of oral therapy are mainly gastrointestinal, including nausea, dyspepsia, constipation or diarrhoea [49, 50, 81]. They are usually not severe, but are directly proportional to the amount of iron ingested. Some individuals with an existing tendency to constipation benefit from drinking additional fruit juice to prevent heavy constipation. Otherwise compliance may be seriously affected.
Intravenous iron
Only when oral therapy repeatedly fails or immediate restoration is needed should intravenous therapy be considered. At the moment, two preparations are available in Switzerland, one containing a Fe3+-saccharose complex [82] and the other a Fe3+-carboxymaltose complex [83]. The dosage is dependent on the severity of the iron deficiency. In one administration, 200 mg Fe-saccharose, or 500 to 1000 mg Fe-carboxymaltose can usually be given [81]. In the case of IDA, the iron deficit to be replaced may be calculated with the Ganzoni equation: total iron deficit = weight (kg) × (target Hb – actual Hb) (g/l) ×2.4 + iron stores (mg); as iron stores in patients weighing >35 kg, 500 mg should be used. This equation helps to estimate the amount of iron needed.
The main advantage of intravenous therapy is the immediate correction of the iron deficiency and restoration of the empty iron stores. Generally, compliance with intravenous iron supplements is good. Side effects may include transient disturbance of taste, headache, dizziness, myalgia and fever. Serious adverse reactions, such as hypotonic and anaphylactoid reactions, tachycardia and arrhythmia, dyspnoea and bronchospasm may be observed, although they are very rare [81]. Moreover, transient and usually asymptomatic hypophosphataemia is frequently observed after the administration of Fe-carboxymaltose. It is still under discussion whether hypophosphataemia may possibly be a cofactor for cardiac events [84]. Nowadays, serious side effects are rare. For Fe-saccharose and Fe-carboxymaltose, no fatal outcome has been reported in Switzerland. Internationally there has been just one fatality: an already severely ill patient treated with Fe-carboxymaltose. This is in contrast to the previously used iron-dextran products, which have a much higher rate of serious and fatal side effects. In 2013, another type of intravenous iron, Fe-oxytol, was withdrawn because of severe hypersensitivity reactions in four patients, including one with fatal outcome, observed within 9 months after approval by the licensing authorities in Switzerland [85, 86]. Even with the use of the new preparations, serious adverse reactions cannot be excluded, and the administration of intravenous iron preparations is only recommended in settings where resuscitation skills are available and patient observation for 30 minutes after the end of iron administration can be guaranteed [82, 83]. In general, the frequency of side effects seems to be lower when the intravenous iron is administered as an infusion instead of a slow bolus injection. Importantly, the exact dilution given by the manufacturer needs to be followed.
Control of adequate iron stores and prevention of excessive treatment
To monitor the efficacy of therapeutic measures we recommend repeating the basic blood tests 6 to 8 weeks after the start of the nutritional measures, oral therapy or intravenous iron administration.
Depending on the blood results, therapy will be continued or modified with the aim to reach or keep iron stores within the normal range. Treatment approaches may be combined. Athletes with recurrent low iron stores may benefit from intermittent oral substitution to preserve iron stores (e.g., two iron tablets per week or daily supplementation with 14 or 28 mg), as well as nutritional counselling. For vegetarians, a similar therapeutic approach to prevent iron deficiency may be recommended: 28 to 50 mg elemental iron three times per week instead of meat intake, or daily supplementation with at least 14 or 28 mg of iron, will usually cover iron demand.
As iron homeostasis is exclusively and meticulously controlled by iron uptake through the intestinal tract [87], and as there is no pathway to eliminate iron in the event of overload, iron supplementation should always be careful. In extreme cases, chronic overload may lead to secondary haemochromatosis. Furthermore, excessive supplementation of oral or intravenous iron is thought to increase oxidative stress and production of free radicals [88, 89], and oxidative stress is suggested to play a role in causing cancer [90, 91]. This must be critically appraised as the intention of treatment for iron deficiency is still “first do no harm” [92].
It has been shown in mice that oral iron supplementation enhances colonic tumour development [93]. Data in humans suggest that iron may increase the risk of colorectal cancer [94]. A recent meta-analysis showed on the one hand a tendency toward a positive association between high intake of haem iron and cancer risk; on the other hand, high levels of biomarkers of iron stores implied a low cancer risk [95]. Further prospective and experimental studies are needed to evaluate the possible influence of iron in carcinogenesis.
Iron deficiency in children and adolescents
Total iron requirements in children and adolescents are markedly increased because of additional needs for the expansion of the total blood volume and mean haemoglobin mass, as well as for the increase in lean body mass during growth [96]. In adolescent females the onset of menarche is associated with an increased requirement for iron. The mean total iron requirement for adolescents reaches 1.8 mg per day for boys and 2.2 mg per day for girls (in females with heavy periods it is considerably more), which corresponds to more than double that in the preadolescent period [15, 97]. Haematological normal values for children and adolescents are different from adults and this should always be considered. We recommend defining the lower level of normal ferritin as 15 µg/l for children aged between 6 and 12 years, 20 µg/l between 12 and 15 years and 30 µg/l for 15- to 18-year-old adolescents [69].
As in adults, in a case of NAID, dietary counselling is the first step, often combined with oral therapy. Iron requirements (RDA) are 8 mg per day for 9- to 13-year-old children, and 11 mg per day for male and 15 mg per day for female adolescents older than 13 years. Careful management, especially of the menstruating teenage girl and the vegetarian athlete, is warranted [19, 75]. If the iron deficiency results in IDMH or IDA, further supplementation should be considered. Comparable to the therapy in adults, either Fe2+ or Fe3+ preparations can be used for oral substitution. The dosage for both preparations is 3 mg/kg/d up to a maximum of 50 mg elemental iron for 3 months in two or three doses per day. The Fe2+ preparation is recommended as medication of choice owing to a better bioavailability. As in adults, measurement of haemoglobin, red cell indices, ferritin and CRP after 6–8 weeks of treatment is necessary in order to observe any response to and compliance with treatment.
Conclusions and recommendations
Iron deficiency is frequent and relevant as all stages of iron deficiency, IDA, IDMH and NAID affect physical performance and cognition.
To diagnose iron deficiency haemoglobin, haematocrit, % HYPO, MCV, MCH and ferritin are first-line parameters to assess. For a valid interpretation of the results it is necessary to exclude acute phase reactions that may interfere, such as training sessions and infectious diseases (patient history and measurement of CRP). In unclear situations a second measurement of the same parameters or the additional measurement of zinc protoporphyrin, soluble transferrin receptor and transferrin saturation may be helpful.
Ferritin values below <15 µg/l are very specific for empty iron stores. Ferritin values from 15 to 30 µg/l correspond to low iron stores. A ferritin value of 30 µg/l should be taken as a reasonable cut-off for adult men and women and older adolescents (15 years and older). For younger adolescents aged from 12 to 15 years a cut-off of 20 µg/l and for children from 6 to 12 years a cut-off of 15 µg/l are recommended. Every case of unexplained or relapsing iron deficiency anaemia warrants an extended diagnostic work up.
Therapy of iron deficiency consists of nutritional counselling, including a sufficient energy intake and haem iron intake (meat, poultry, fish) five times per week with the addition of legumes and green vegetables (e.g., spinach, fennel), usually combined with oral iron supplementation. Oral iron preparations at a dosage of 28 to 50 mg of elemental iron daily are appropriate, as the nonserious but disturbing gastrointestinal side effects are related to iron dosage and preparation. Consider enhancers (vitamin C) and recommend avoidance of inhibitors (coffee, black tea, phytates, calcium) of iron uptake to increase absorption. Patients with repeatedly low ferritin values benefit from intermittent oral substitution to preserve iron stores, for example two iron tablets per week as maintenance therapy. Only in selected cases with severe incompatibility with oral therapy, a concomitant disease (e.g., depression) or repeated and plausible nonresponse to oral treatment, intravenous iron therapy may be considered.
Long-term daily oral iron intake or intravenous supplementation in the presence of normal or high ferritin values is not recommended and may be harmful.
Parts of this article have already been published in: Clénin G, Cordes M, Huber A, Schumacher YO, Noack P, Scales J, Kriemler S. Iron deficiency in sports – definition, influence on performance and therapy. Swiss Med Wkly. 2015;145:w14196.
Acknowledgements
We thank the author team of the consensus paper of the Swiss Society of Sports Medicine “Iron deficiency in sports - definition, influence on performance and therapy” recently published in this journal: Dr Mareike Cordes, Prof. Dr Andreas Huber, Prof. Dr Yorck Olaf Schumacher, Dr Patrik Noack, Dr John Scales and Prof. Dr Susi Kriemler. This consensus paper and the contribution of all named authors was the foundation for this “current opinion” article.
German Clénin, Sportmedizinisches Zentrum Ittigen bei Bern Haus des Sports, CH-3063 Ittigen, german.clenin[at]smzbi.ch
References
1WHO | Micronutrients [Internet]. WHO. [cited 2016 Feb 3]. Available from: http://www.who.int/nutrition/topics/micronutrients/en/
2Iron deficiency anaemia: assessment, prevention and control A guide for programme managers.partie i. 6 - WHO_NHD_01.3.pdf [Internet]. [cited 2016 Feb 3]. Available from: http://apps.who.int/iris/bitstream/10665/66914/1/WHO_NHD_01.3.pdf?ua=1
3WHO. The global prevalence of anaemia in 2011. Geneva: World Health Organization; 2015.WHO. The global prevalence of anaemia in 2011. Geneva: World Health Organization; 2015.9789241564960_eng.pdf [Internet]. [cited 2016 Feb 3]. Available from: http://apps.who.int/iris/bitstream/10665/177094/1/9789241564960_eng.pdf?ua=1
4
Fayet-Moore
F
,
Petocz
P
,
Samman
S
. Micronutrient status in female university students: iron, zinc, copper, selenium, vitamin B12 and folate. Nutrients. 2014;6(11):5103–16.https://doi.org/10.3390/nu6115103
5
Milman
N
. Serum ferritin in Danes: studies of iron status from infancy to old age, during blood donation and pregnancy. Int J Hematol. 1996;63(2):103–35.https://doi.org/10.1016/0925-5710(95)00426-2
6
Milman
N
,
Ulrik
CS
,
Graudal
N
,
Jordal
R
. Iron status in young Danes. Evaluation by serum ferritin and haemoglobin in a population survey of 634 individuals aged 14-23 yr. Eur J Haematol. 1997;58(3):160–6.https://doi.org/10.1111/j.1600-0609.1997.tb00942.x
7
Milman
N
,
Byg
KE
,
Ovesen
L
. Iron status in Danes 1994. II: Prevalence of iron deficiency and iron overload in 1319 Danish women aged 40-70 years. Influence of blood donation, alcohol intake and iron supplementation. Ann Hematol. 2000;79(11):612–21.https://doi.org/10.1007/s002770000209
8
Schleiffenbaum
BE
,
Schaer
DJ
,
Burki
D
,
Viollier
A-F
,
Viollier
E
,
Stettler
ER
, et al.
Unexpected high prevalence of metabolic disorders and chronic disease among young male draftees--the Swiss Army XXI experience. Swiss Med Wkly. 2006;136(11-12):175–84.
9
Andersson
M
,
Egli
IM
,
Zimmerman
MB
. Eisenmangel. Schweiz Z für Ernährungsmedizin.
2010;2010(1):13–8. Article in German.
10
Looker
AC
,
Dallman
PR
,
Carroll
MD
,
Gunter
EW
,
Johnson
CL
. Prevalence of iron deficiency in the United States. JAMA. 1997;277(12):973–6.https://doi.org/10.1001/jama.1997.03540360041028
11
Cogswell
ME
,
Looker
AC
,
Pfeiffer
CM
,
Cook
JD
,
Lacher
DA
,
Beard
JL
, et al.
Assessment of iron deficiency in US preschool children and nonpregnant females of childbearing age: National Health and Nutrition Examination Survey 2003-2006. Am J Clin Nutr. 2009;89(5):1334–42.https://doi.org/10.3945/ajcn.2008.27151
12
Ganz
T
. Molecular control of iron transport. J Am Soc Nephrol. 2007;18(2):394–400.https://doi.org/10.1681/ASN.2006070802
13Petrides P, Löffler G. Eisen. In: Chemie und Pathobiochemie. 6.Auflage ed. Berlin Heidelberg: Springer Verlag; 1998. p. 416 ff.
14
Ganz
T
,
Nemeth
E
. Iron metabolism: interactions with normal and disordered erythropoiesis. Cold Spring Harb Perspect Med. 2012;2(5):a011668.https://doi.org/10.1101/cshperspect.a011668
15Hallberg L. Iron requirements, iron balance and iron deficiency in menstruating and pregnant women. In: Iron Nutrition in Health and Disease. John Libbey & Co; London, UK; 1996. p. 165–82.
16
Kim
A
,
Nemeth
E
. New insights into iron regulation and erythropoiesis. Curr Opin Hematol. 2015;22(3):199–205.https://doi.org/10.1097/MOH.0000000000000132
17Yip R. Iron. In: Present Knowledge in Nutrition. 8th ed. Washington DC: ILSI Press; 2001. p. 311–28.
18
Latunde-Dada
GO
. Iron metabolism in athletes--achieving a gold standard. Eur J Haematol. 2013;90(1):10–5.https://doi.org/10.1111/ejh.12026
19
Sandström
G
,
Börjesson
M
,
Rödjer
S
. Iron deficiency in adolescent female athletes - is iron status affected by regular sporting activity?
Clin J Sport Med. 2012;22(6):495–500.https://doi.org/10.1097/JSM.0b013e3182639522
20
Camaschella
C
,
Pagani
A
,
Nai
A
,
Silvestri
L
. The mutual control of iron and erythropoiesis. Int J Lab Hematol. 2016;38(Suppl 1):20–6.https://doi.org/10.1111/ijlh.12505
21
Kautz
L
,
Jung
G
,
Valore
EV
,
Rivella
S
,
Nemeth
E
,
Ganz
T
. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet. 2014;46(7):678–84.https://doi.org/10.1038/ng.2996
22
Camaschella
C
. Iron-deficiency anemia. N Engl J Med. 2015;372(19):1832–43.https://doi.org/10.1056/NEJMra1401038
23
Davies
KJ
,
Donovan
CM
,
Refino
CJ
,
Brooks
GA
,
Packer
L
,
Dallman
PR
. Distinguishing effects of anemia and muscle iron deficiency on exercise bioenergetics in the rat. Am J Physiol. 1984;246(6 Pt 1):E535–43.
24
Finch
CA
,
Miller
LR
,
Inamdar
AR
,
Person
R
,
Seiler
K
,
Mackler
B
. Iron deficiency in the rat. Physiological and biochemical studies of muscle dysfunction. J Clin Invest. 1976;58(2):447–53.https://doi.org/10.1172/JCI108489
25
Hallberg
L
,
Bengtsson
C
,
Lapidus
L
,
Lindstedt
G
,
Lundberg
PA
,
Hultén
L
. Screening for iron deficiency: an analysis based on bone-marrow examinations and serum ferritin determinations in a population sample of women. Br J Haematol. 1993;85(4):787–98.https://doi.org/10.1111/j.1365-2141.1993.tb03225.x
26
Thomason
RW
,
Almiski
MS
. Evidence that stainable bone marrow iron following parenteral iron therapy does not correlate with serum iron studies and may not represent readily available storage iron. Am J Clin Pathol. 2009;131(4):580–5.https://doi.org/10.1309/AJCPBAY9KRZF8NUC
27
Magnusson
B
,
Hallberg
L
,
Rossander
L
,
Swolin
B
. Iron metabolism and “sports anemia”. I. A study of several iron parameters in elite runners with differences in iron status. Acta Med Scand. 1984;216(2):149–55.https://doi.org/10.1111/j.0954-6820.1984.tb03786.x
28
Krayenbuehl
P-A
,
Battegay
E
,
Breymann
C
,
Furrer
J
,
Schulthess
G
. Intravenous iron for the treatment of fatigue in nonanemic, premenopausal women with low serum ferritin concentration. Blood. 2011;118(12):3222–7.https://doi.org/10.1182/blood-2011-04-346304
29
Verdon
F
,
Burnand
B
,
Stubi
C-LF
,
Bonard
C
,
Graff
M
,
Michaud
A
, et al.
Iron supplementation for unexplained fatigue in non-anaemic women: double blind randomised placebo controlled trial. BMJ. 2003;326(7399):1124.https://doi.org/10.1136/bmj.326.7399.1124
30
Vaucher
P
,
Druais
P-L
,
Waldvogel
S
,
Favrat
B
. Effect of iron supplementation on fatigue in nonanemic menstruating women with low ferritin: a randomized controlled trial. CMAJ. 2012;184(11):1247–54.https://doi.org/10.1503/cmaj.110950
31
Burden
RJ
,
Pollock
N
,
Whyte
GP
,
Richards
T
,
Moore
B
,
Busbridge
M
, et al.
Effect of Intravenous Iron on Aerobic Capacity and Iron Metabolism in Elite Athletes. Med Sci Sports Exerc. 2015;47(7):1399–407.https://doi.org/10.1249/MSS.0000000000000568
32
Garvican
LA
,
Saunders
PU
,
Cardoso
T
,
Macdougall
IC
,
Lobigs
LM
,
Fazakerley
R
, et al.
Intravenous iron supplementation in distance runners with low or suboptimal ferritin. Med Sci Sports Exerc. 2014;46(2):376–85.https://doi.org/10.1249/MSS.0b013e3182a53594
33
DellaValle
DM
,
Haas
JD
. Iron supplementation improves energetic efficiency in iron-depleted female rowers. Med Sci Sports Exerc. 2014;46(6):1204–15.https://doi.org/10.1249/MSS.0000000000000208
34
Waldvogel
S
,
Pedrazzini
B
,
Vaucher
P
,
Bize
R
,
Cornuz
J
,
Tissot
J-D
, et al.
Clinical evaluation of iron treatment efficiency among non-anemic but iron-deficient female blood donors: a randomized controlled trial. BMC Med. 2012;10(1):8.https://doi.org/10.1186/1741-7015-10-8
35
McClung
JP
,
Karl
JP
,
Cable
SJ
,
Williams
KW
,
Nindl
BC
,
Young
AJ
, et al.
Randomized, double-blind, placebo-controlled trial of iron supplementation in female soldiers during military training: effects on iron status, physical performance, and mood. Am J Clin Nutr. 2009;90(1):124–31.https://doi.org/10.3945/ajcn.2009.27774
36
Hinton
PS
,
Sinclair
LM
. Iron supplementation maintains ventilatory threshold and improves energetic efficiency in iron-deficient nonanemic athletes. Eur J Clin Nutr. 2007;61(1):30–9.https://doi.org/10.1038/sj.ejcn.1602479
37
Brownlie
T, 4th
,
Utermohlen
V
,
Hinton
PS
,
Haas
JD
. Tissue iron deficiency without anemia impairs adaptation in endurance capacity after aerobic training in previously untrained women. Am J Clin Nutr. 2004;79(3):437–43.
38
Bruner
AB
,
Joffe
A
,
Duggan
AK
,
Casella
JF
,
Brandt
J
. Randomised study of cognitive effects of iron supplementation in non-anaemic iron-deficient adolescent girls. Lancet. 1996;348(9033):992–6.https://doi.org/10.1016/S0140-6736(96)02341-0
39
Falkingham
M
,
Abdelhamid
A
,
Curtis
P
,
Fairweather-Tait
S
,
Dye
L
,
Hooper
L
. The effects of oral iron supplementation on cognition in older children and adults: a systematic review and meta-analysis. Nutr J. 2010;9(1):4.https://doi.org/10.1186/1475-2891-9-4
40
Baumgartner
J
,
Smuts
CM
,
Malan
L
,
Kvalsvig
J
,
van Stuijvenberg
ME
,
Hurrell
RF
, et al.
Effects of iron and n-3 fatty acid supplementation, alone and in combination, on cognition in school children: a randomized, double-blind, placebo-controlled intervention in South Africa. Am J Clin Nutr. 2012;96(6):1327–38.https://doi.org/10.3945/ajcn.112.041004
41
Domellöf
M
,
Thorsdottir
I
,
Thorstensen
K
. Health effects of different dietary iron intakes: a systematic literature review for the 5th Nordic Nutrition Recommendations. Food Nutr Res. 2013;57(1):21667.https://doi.org/10.3402/fnr.v57i0.21667
42
Greig
AJ
,
Patterson
AJ
,
Collins
CE
,
Chalmers
KA
. Iron deficiency, cognition, mental health and fatigue in women of childbearing age: a systematic review. J Nutr Sci. 2013;2:e14.https://doi.org/10.1017/jns.2013.7
43
Leonard
AJ
,
Chalmers
KA
,
Collins
CE
,
Patterson
AJ
. A study of the effects of latent iron deficiency on measures of cognition: a pilot randomised controlled trial of iron supplementation in young women. Nutrients. 2014;6(6):2419–35.https://doi.org/10.3390/nu6062419
44
Murray-Kolb
LE
,
Beard
JL
. Iron treatment normalizes cognitive functioning in young women. Am J Clin Nutr. 2007;85(3):778–87.
45Effects of Iron Treatment on Cognitive Performance and Working Memory in Non-anemic. Iron-deficient Girls.NZJP-Vol311-2002-3-Lambert.pdf [Internet]. [cited 2016 Feb 3]. Available from: http://www.psychology.org.nz/wp-content/uploads/NZJP-Vol311-2002-3-Lambert.pdf
46
Lynn
R
,
Harland
EP
. A positive effect of iron supplementation on the IQS of iron deficient children. Pers Individ Dif. 1998;24(6):883–5. doi:.https://doi.org/10.1016/S0191-8869(97)00219-5
47
Groner
JA
,
Holtzman
NA
,
Charney
E
,
Mellits
ED
. A randomized trial of oral iron on tests of short-term memory and attention span in young pregnant women. J Adolesc Health Care. 1986;7(1):44–8.https://doi.org/10.1016/S0197-0070(86)80094-8
48
Clénin
G
,
Cordes
M
,
Huber
A
,
Schumacher
YO
,
Noack
P
,
Scales
J
, et al.
Iron deficiency in sports - definition, influence on performance and therapy. Swiss Med Wkly. 2015;145:w14196.
https://dx.doi.org/10.4414/smw.2015.14196
49
Clénin
G.
Eisen im Sport - oft zu wenig, gelegentlich aber auch zu viel. Schweiz Z für Ernährungsmedizin. 2006;2:21–5. Article in German.
50
Mettler
S
. Ferrum - ein Mineralstoff im Sport. Schweiz Z für Sportmed und Sportraumatologie.
2004;52:105–14. Article in German.
51
Lamanca
JJ
,
Haymes
EM
. Effects of low ferritin concentration on endurance performance. Int J Sport Nutr. 1992;2(4):376–85.https://doi.org/10.1123/ijsn.2.4.376
52
Yu
D
,
Huo
J
,
Xie
L
,
Wang
L
. [Meta-analysis of studies on cut-off value of serum ferritin for identifying iron deficiency]. Wei Sheng Yan Jiu. 2013;42(2):228–35. Article in Chinese.
53
Pitsis
GC
,
Fallon
KE
,
Fallon
SK
,
Fazakerley
R
. Response of soluble transferrin receptor and iron-related parameters to iron supplementation in elite, iron-depleted, nonanemic female athletes. Clin J Sport Med. 2004;14(5):300–4.https://doi.org/10.1097/00042752-200409000-00009
54
Fallon
KE
. Utility of hematological and iron-related screening in elite athletes. Clin J Sport Med. 2004;14(3):145–52.https://doi.org/10.1097/00042752-200405000-00007
55
Fallon
KE
. Screening for haematological and iron-related abnormalities in elite athletes-analysis of 576 cases. J Sci Med Sport. 2008;11(3):329–36.https://doi.org/10.1016/j.jsams.2007.02.017
56
Macdougall
IC
. What is the most appropriate strategy to monitor functional iron deficiency in the dialysed patient on rhEPO therapy? Merits of percentage hypochromic red cells as a marker of functional iron deficiency. Nephrol Dial Transplant. 1998;13(4):847–9.https://doi.org/10.1093/ndt/13.4.847
57
d’Onofrio
G
,
Zini
G
,
Ricerca
BM
,
Mancini
S
,
Mango
G
. Automated measurement of red blood cell microcytosis and hypochromia in iron deficiency and beta-thalassemia trait. Arch Pathol Lab Med. 1992;116(1):84–9.
58
Urrechaga
E
,
Borque
L
,
Escanero
JF
. Percentage of hypochromic erythrocytes as a potential marker of iron availability. Clin Chem Lab Med. 2011;50(4):685–7.
59
Hinchliffe
RF
,
Vora
AJ
,
Lennard
L
. An assessment of methods used in the investigation of iron status: findings in a population of young British South Asian children. J Clin Pathol. 2016;69(4):345–51.
60
Thomas
C
,
Thomas
L
. Biochemical markers and hematologic indices in the diagnosis of functional iron deficiency. Clin Chem. 2002;48(7):1066–76.
61
Brugnara
C
,
Laufer
MR
,
Friedman
AJ
,
Bridges
K
,
Platt
O
. Reticulocyte hemoglobin content (CHr): early indicator of iron deficiency and response to therapy. Blood. 1994;83(10):3100–1.
62
Brugnara
C
,
Zurakowski
D
,
DiCanzio
J
,
Boyd
T
,
Platt
O
. Reticulocyte hemoglobin content to diagnose iron deficiency in children. JAMA. 1999;281(23):2225–30.https://doi.org/10.1001/jama.281.23.2225
63
d’Onofrio
G
,
Chirillo
R
,
Zini
G
,
Caenaro
G
,
Tommasi
M
,
Micciulli
G
. Simultaneous measurement of reticulocyte and red blood cell indices in healthy subjects and patients with microcytic and macrocytic anemia. Blood. 1995;85(3):818–23.
64
Franck
S
,
Linssen
J
,
Messinger
M
,
Thomas
L
. Potential utility of Ret-Y in the diagnosis of iron-restricted erythropoiesis. Clin Chem. 2004;50(7):1240–2.https://doi.org/10.1373/clinchem.2004.030254
65
Schumacher
YO
,
Schmid
A
,
König
D
,
Berg
A
. Effects of exercise on soluble transferrin receptor and other variables of the iron status. Br J Sports Med. 2002;36(3):195–9.https://doi.org/10.1136/bjsm.36.3.195
66
Magge
H
,
Sprinz
P
,
Adams
WG
,
Drainoni
M-L
,
Meyers
A
. Zinc protoporphyrin and iron deficiency screening: trends and therapeutic response in an urban pediatric center. JAMA Pediatr. 2013;167(4):361–7.https://doi.org/10.1001/jamapediatrics.2013.751
67
Baart
AM
,
van Noord
PAH
,
Vergouwe
Y
,
Moons
KGM
,
Swinkels
DW
,
Wiegerinck
ET
, et al.
High prevalence of subclinical iron deficiency in whole blood donors not deferred for low hemoglobin. Transfusion. 2013;53(8):1670–7.https://doi.org/10.1111/j.1537-2995.2012.03956.x
68
Newlin
MK
,
Williams
S
,
McNamara
T
,
Tjalsma
H
,
Swinkels
DW
,
Haymes
EM
. The effects of acute exercise bouts on hepcidin in women. Int J Sport Nutr Exerc Metab. 2012;22(2):79–88.https://doi.org/10.1123/ijsnem.22.2.79
69
Herklotz
R
,
Lüthi
U
,
Ottiger
C
,
Huber
AR
. Referenzbereiche in der Hämatologie. Ther Umsch Rev Thérapeutique. 2006;63(1):5–24. Article in German. https://doi.org/10.1024/0040-5930.63.1.5
70Bothwell T, Charlton R, Cook J, Finch C. Iron metabolism in man. Oxford UK: Blackwell Scientific Publications; 1979.
71
Miret
S
,
Simpson
RJ
,
McKie
AT
. Physiology and molecular biology of dietary iron absorption. Annu Rev Nutr. 2003;23(1):283–301.https://doi.org/10.1146/annurev.nutr.23.011702.073139
72
Zimmermann
MB
,
Biebinger
R
,
Egli
I
,
Zeder
C
,
Hurrell
RF
. Iron deficiency up-regulates iron absorption from ferrous sulphate but not ferric pyrophosphate and consequently food fortification with ferrous sulphate has relatively greater efficacy in iron-deficient individuals. Br J Nutr. 2011;105(8):1245–50.https://doi.org/10.1017/S0007114510004903
73
Monsen
ER
. Iron nutrition and absorption: dietary factors which impact iron bioavailability. J Am Diet Assoc. 1988;88(7):786–90.
74
Proposed nutrient and energy intakes for the European community: a report of the Scientific Committee for Food of the European community. Nutr Rev. 1993;51(7):209–12.
75Dietary Reference Intakes (DRIs): Estimated Average Requirements for Groups - 5_Summary Table Tables 1-4.pdf [Internet]. [cited 2016 Feb 3]. Available from: https://iom.nationalacademies.org/~/media/Files/Activity%20Files/Nutrition/DRIs/5_Summary%20Table%20Tables%201-4.pdf
76
Hercberg
S
,
Preziosi
P
,
Galan
P
. Iron deficiency in Europe. Public Health Nutr. 2001;4(2B):537–45.https://doi.org/10.1079/PHN2001139
77
Rimon
E
,
Kagansky
N
,
Kagansky
M
,
Mechnick
L
,
Mashiah
T
,
Namir
M
, et al.
Are we giving too much iron? Low-dose iron therapy is effective in octogenarians. Am J Med. 2005;118(10):1142–7.https://doi.org/10.1016/j.amjmed.2005.01.065
78
Zimmermann
MB
,
Troesch
B
,
Biebinger
R
,
Egli
I
,
Zeder
C
,
Hurrell
RF
. Plasma hepcidin is a modest predictor of dietary iron bioavailability in humans, whereas oral iron loading, measured by stable-isotope appearance curves, increases plasma hepcidin. Am J Clin Nutr. 2009;90(5):1280–7.https://doi.org/10.3945/ajcn.2009.28129
79
DellaValle
DM
. Iron supplementation for female athletes: effects on iron status and performance outcomes. Curr Sports Med Rep. 2013;12(4):234–9.https://doi.org/10.1249/JSR.0b013e31829a6f6b
80
Cancelo-Hidalgo
MJ
,
Castelo-Branco
C
,
Palacios
S
,
Haya-Palazuelos
J
,
Ciria-Recasens
M
,
Manasanch
J
, et al.
Tolerability of different oral iron supplements: a systematic review. Curr Med Res Opin. 2013;29(4):291–303.https://doi.org/10.1185/03007995.2012.761599
81
Arzneimittelkompendium der Schweiz. Documed; 2012.
82Arzneimittelinformation über Fe-Saccharose (Venofer) [Internet]. [cited 2016 Feb 3]. Available from: http://www.swissmedicinfo.ch/
83Arzneimittelinformation über Fe-Carboxymaltose (Ferinject) [Internet]. [cited 2016 Feb 3]. Available from: http://www.swissmedicinfo.ch/
84Eisencarboxymaltose. Ritzmann P. Pharmakritik 2010.pk08-10.pdf [Internet]. [cited 2016 Feb 3]. Available from: http://www.infomed.ch/attachments/pk08-10.pdf
85Rienso®, Lösung zur intravenösen Injektion (Ferumoxytol) - Swissmedic - [Internet]. [cited 2016 Feb 3]. Available from: https://www.swissmedic.ch/zulassungen/00153/00189/00200/00497/index.html?lang=de
86Rienso, Lösung zur intravenösen Injektion - Swissmedic - [Internet]. [cited 2016 Feb 3]. Available from: https://www.swissmedic.ch/marktueberwachung/00135/00166/00707/index.html?lang=de
87Demarmels Biasiutti F. SMF Artikel - Schweizerisches Medizin-Forum - Die Regulation des Eisenstoffwechsels [Internet]. 2009 [cited 2016 Feb 3]. Available from: http://medicalforum.ch/index.php?id=644&tx_topiccollection_tccollection%5Baction%5D=show&tx_topiccollection_tccollection%5Bcontroller%5D=Article&cHash=9b2c579bf6a9ef0a06b8f2947b872b83&tx_topiccollection_tccollection%5Barticle%5D=2085
88
Koskenkorva-Frank
TS
,
Weiss
G
,
Koppenol
WH
,
Burckhardt
S
. The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free Radic Biol Med. 2013;65:1174–94.https://doi.org/10.1016/j.freeradbiomed.2013.09.001
89
Kohgo
Y
,
Ikuta
K
,
Ohtake
T
,
Torimoto
Y
,
Kato
J
. Body iron metabolism and pathophysiology of iron overload. Int J Hematol. 2008;88(1):7–15.https://doi.org/10.1007/s12185-008-0120-5
90
Steinboeck
F
,
Hubmann
M
,
Bogusch
A
,
Dorninger
P
,
Lengheimer
T
,
Heidenreich
E
. The relevance of oxidative stress and cytotoxic DNA lesions for spontaneous mutagenesis in non-replicating yeast cells. Mutat Res. 2010;688(1-2):47–52.https://doi.org/10.1016/j.mrfmmm.2010.03.006
91
Nowsheen
S
,
Wukovich
RL
,
Aziz
K
,
Kalogerinis
PT
,
Richardson
CC
,
Panayiotidis
MI
, et al.
Accumulation of oxidatively induced clustered DNA lesions in human tumor tissues. Mutat Res. 2009;674(1-2):131–6.https://doi.org/10.1016/j.mrgentox.2008.09.010
92
Zoller
H
,
Vogel
W
. Iron supplementation in athletes--first do no harm. Nutrition. 2004;20(7-8):615–9.https://doi.org/10.1016/j.nut.2004.04.006
93
Chua
ACG
,
Klopcic
BRS
,
Ho
DS
,
Fu
SK
,
Forrest
CH
,
Croft
KD
, et al.
Dietary iron enhances colonic inflammation and IL-6/IL-11-Stat3 signaling promoting colonic tumor development in mice. PLoS One. 2013;8(11):e78850.https://doi.org/10.1371/journal.pone.0078850
94
Wurzelmann
JI
,
Silver
A
,
Schreinemachers
DM
,
Sandler
RS
,
Everson
RB
. Iron intake and the risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 1996;5(7):503–7.
95
Fonseca-Nunes
A
,
Jakszyn
P
,
Agudo
A
. Iron and cancer risk--a systematic review and meta-analysis of the epidemiological evidence. Cancer Epidemiol Biomarkers Prev. 2014;23(1):12–31.https://doi.org/10.1158/1055-9965.EPI-13-0733
96Fairweather-Tait S. Iron requirements and prevalence of iron deficiency. Adolescents - an overview. In: Iron Nutrition in Health and Disease. John Libbey & Co; London, UK; 1996. p. 137–48.
97
Beard
JL
. Iron requirements in adolescent females. J Nutr. 2000;130(2S, Suppl):440S–2S.


